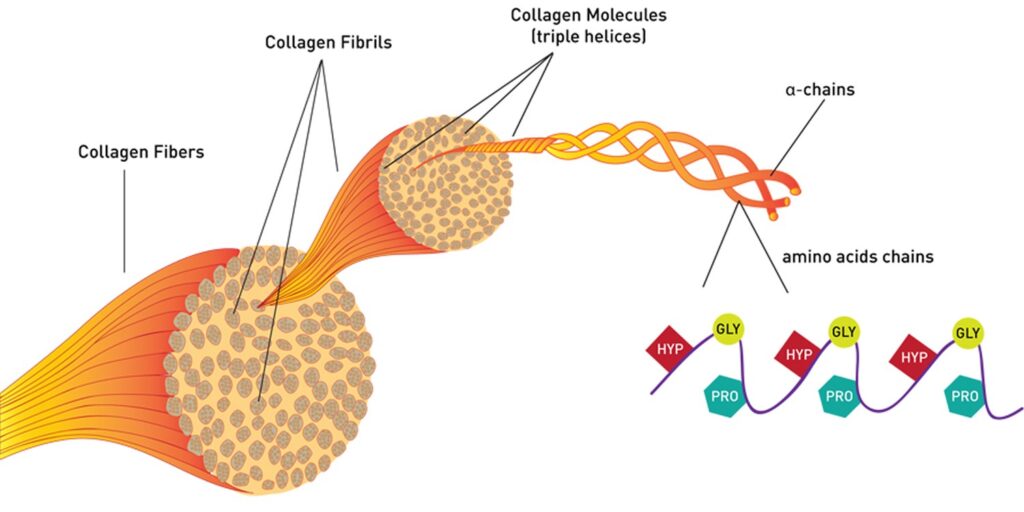
case study Situation Cell isolation from tissue or recovery after in vitro cell culture often requires collagenase or protease enzymes. The enzyme-mediated steps of cell isolation or recovery methods are critical process parameters of the therapeutic cell manufacturing process. However, in most cases, the critical quality attributes of these enzymes are not defined. Also, steps
Your Second Source of Cell Isolation Enzymes