
Francis Dwulet and I co-founded VitaCyte 20 years ago (17 September 2003) to continue the development of improved tissue dissociation enzymes for cell isolation. The key roles we played in developing the Liberase Purified Enzyme Blend product line at Boehringer Mannheim Biochemicals’ (BMB) R&D facility in Indianapolis from 1993 to 1998 preordained the formation of VitaCyte to extend and improve the purification and characterization of collagenase and protease enzymes used for cell isolation. I was the project leader, and Francis was responsible for identifying and purifying the key enzymes that freed islets from human pancreatic tissue. Eight other individuals contributed to the project’s success and were driven by the potential contribution it would make to the diabetes community. Francis, David Waters, and Marilyn Smith focused on enzyme purification, John Gill and Bernie Ellis on enzyme characterization, and Terry Fetterhoff, Tom Cavanagh, Kathleen Wile, and Mary Jo Wright assessed the ability of the purified enzyme mixtures first to recover porcine islets, then human islets.
Ten people worked on the project because there was minimal knowledge of the enzymes responsible for tissue dissociation in crude Clostridium histolyticum collagenase. Van Wart’s group published many reports that described the purification and characterization of these enzymes.(1) They reported that seven different molecular forms of C. histolyticum collagenase could be isolated from Worthington crude collagenase products. One publication noted that the multiple forms could be explained by proteolysis of intact collagenases, expression by different genes, or differential processing of mRNA used to express each form of collagenase.(2) Two other investigators showed that when collagenase and protease enzymes were isolated from a “good” lot of crude collagenase, then recombined, this mixture gave results similar to those isolations that used the same good lot of collagenase.(3, 4)
How did Francis sort out the critical information in these reports to develop a plan to identify these active components? The key to success was BMB’s introduction of a new crude collagenase product in 1990, Collagenase P. Unbeknownst to BMB, Collagenase P was different than the crude collagenases manufactured by Sigma or Worthington since it had higher collagenase activity as measured by the Wunsch assay. On screening new lots of Collagenase P, Camillo Ricordi found a higher percentage of these lots (≈ 1/3) could be used for human islet isolation. One lot in particular, Lot 9, gave exceptional results and was used in 22 consecutive islet isolations. (5)
At about the same time, my business colleagues at BMB were concerned that a research-use-only product was used to isolate cells for human cell transplantation. Shaun Lonergan and Len Mascaro met with Camillo Ricordi in Pittsburgh. Camillo convinced these individuals that BMB should fund a project to identify the key enzymes in Collagenase P and manufacture a consistent collagenase product because Boehringer was a “diabetes care company,” and solving this problem could benefit the diabetes community. Shaun organized the visit to Indianapolis. The project was given the green light after Camillo met with senior R&D management.
Camillo’s influence on the human islet transplant community led many laboratories to use Collagenase P for human islet isolation. Francis obtained information from the BMB staff that interacted with these customers. They prepared a list of “good” and “bad” lots of Collagenase P obtained after feedback from human islet isolators who pre-qualified several lots of this product. Francis analyzed them and hypothesized that only collagenase and neutral protease enzymes were the critical enzymes for human islet isolation. He purified the collagenase enzymes but was unsuccessful in purifying C. histolyticum neutral protease since it was unstable after purification. The first test enzyme mixture contained a 60:40 purified class I (C1):class II (C2) C. histolyticum collagenase mixture, a common feature of good Collagenase P lots. Clostripain could not be removed from collagenase, so the activity was adjusted to 7,000 BAEE U of clostripain per bottle in the original version of the Liberase HI product.(6, 7) This collagenase-clostripain mixture was supplemented with Dispase or Thermolysin to replace C. histolyticum neutral protease. Evaluation of these mixtures to recover porcine islets from market-weight pigs showed that either neutral protease worked. The lower cost of Thermolysin led to its inclusion in the Liberase™ HI product.
Several months later, Ray Rajotte at the University of Alberta in Edmonton showed that the purified collagenase-clostripain-Thermolysin mixture could recover human islets from human pancreas. The surprising observations were that digest times were longer and the islets were larger than those obtained when using a Sigma crude collagenase. Several reports confirmed the performance of this product for human islet isolation.(8, 9)
Soon after that, Jonathan Lakey finished his Ph.D. in Ray’s Laboratory and moved to Indianapolis to do postdoctoral work in cryobiology at Methodist Research Institute. He was soon drawn to working with the Liberase project team to assess different enzyme formulations used for porcine islet isolation. He also developed a new enzyme blend, Liberase CI, for canine islet isolation.(7) He was the critical individual who worked with the local organ procurement organization to obtain pancreata for performing human islet isolations at BMB. He participated in the 26 human islet isolations performed at BMB to optimize the enzyme composition of Liberase HI Purified Enzyme Blend.(8)
Liberase HI product was launched by BMB in the US in 1994 and worldwide in 1995. It soon became the “gold standard” for human islet isolation. But there were many questions left unanswered.
Roche purchased Boehringer Mannheim in 1998 and moved the R&D staff from Indianapolis to Berkeley, California. At the end of 2001, BMB’s U.S. R&D operations were closed, and Francis and I were laid off. We began to discuss how we could obtain funding to extend the development of purified collagenase enzymes. We decided to submit a Small Business Innovation Research (SBIR) grant to NIH. Our first attempt failed. We planned to submit a new SBIR grant in December 2003 to respond to an NIH Program Announcement for SBIR/STTR grants targeted to support treatment of type I diabetes. On 17 September 2003, I registered VitaCyte as an LLC in Indiana. In July 2004, VitaCyte received a $500,000, two-year Phase I grant for its application “Tissue Dissociation Enzymes for Islets and Other Cells.” The work on this grant began in October 2004 in a 1000 square foot laboratory across the street from Indiana University Medical Center. The State of Indiana supported VitaCyte by providing an additional $ 75,000 SBIR matching award (match up to $75K for Phase I) to support these efforts.
At that time, Francis was working at a startup in Ann Arbor Michigan, later moving to Eli Lilly in production development followed by employment at Cook Pharmica in a similar position. He joined VitaCyte full time in April 2007. Throughout this time, Francis was intimately involved in the development of methods to improve the purification of Clostridium histolyticum collagenase.
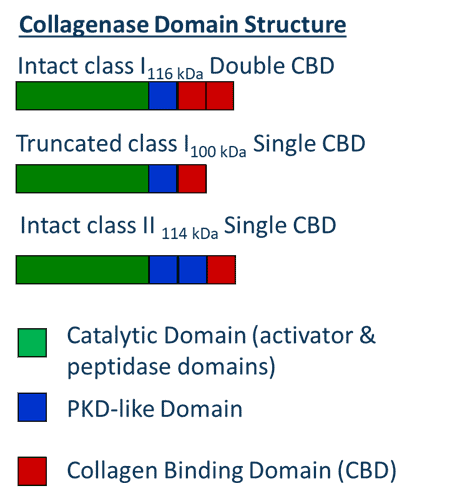
VitaCyte received four Phase I and one Phase II SBIR awards from 2004 to 2009, which laid the foundation for VitaCyte’s commercial success. A key focal point for inclusion in these grant applications was recent information obtained after sequencing C. histolyticum C1 and C2 genes and identification of the functions for each domain.(9) Updated information is presented in the adjacent Figure. Both C1 and C2 collagenases are multidomain proteins. The catalytic domain consists of two amino terminal domains (activator & peptidase domains) that account for about 66% of the ≈ 115 kDa enzymes and are responsible for degrading collagen.(10, 11) Collagen degradation requires two other domains, the PKD (polycystic kidney disease-like protein) and collagen binding domains that bind to collagen’s triple helical structure and play a critical role for collagenases’ ability to degrade collagen.(12) The three molecular forms of collagenase that contain a catalytic domain and at least one collagen binding domain are the only forms that can efficiently degrade native collagen.
The brief review below focuses on the findings from the first and fifth SBIR awards (Phase I & II SBIR award for Tissue Dissociation Enzymes for Islets and Other Cells) and how this contributed to our understanding of how tissue dissociation enzymes free cells from tissue. The Phase I SBIR award was completed in three and one-half years, with the table below summarizing the key findings from the project.
Summary From First Phase I SBIR Award
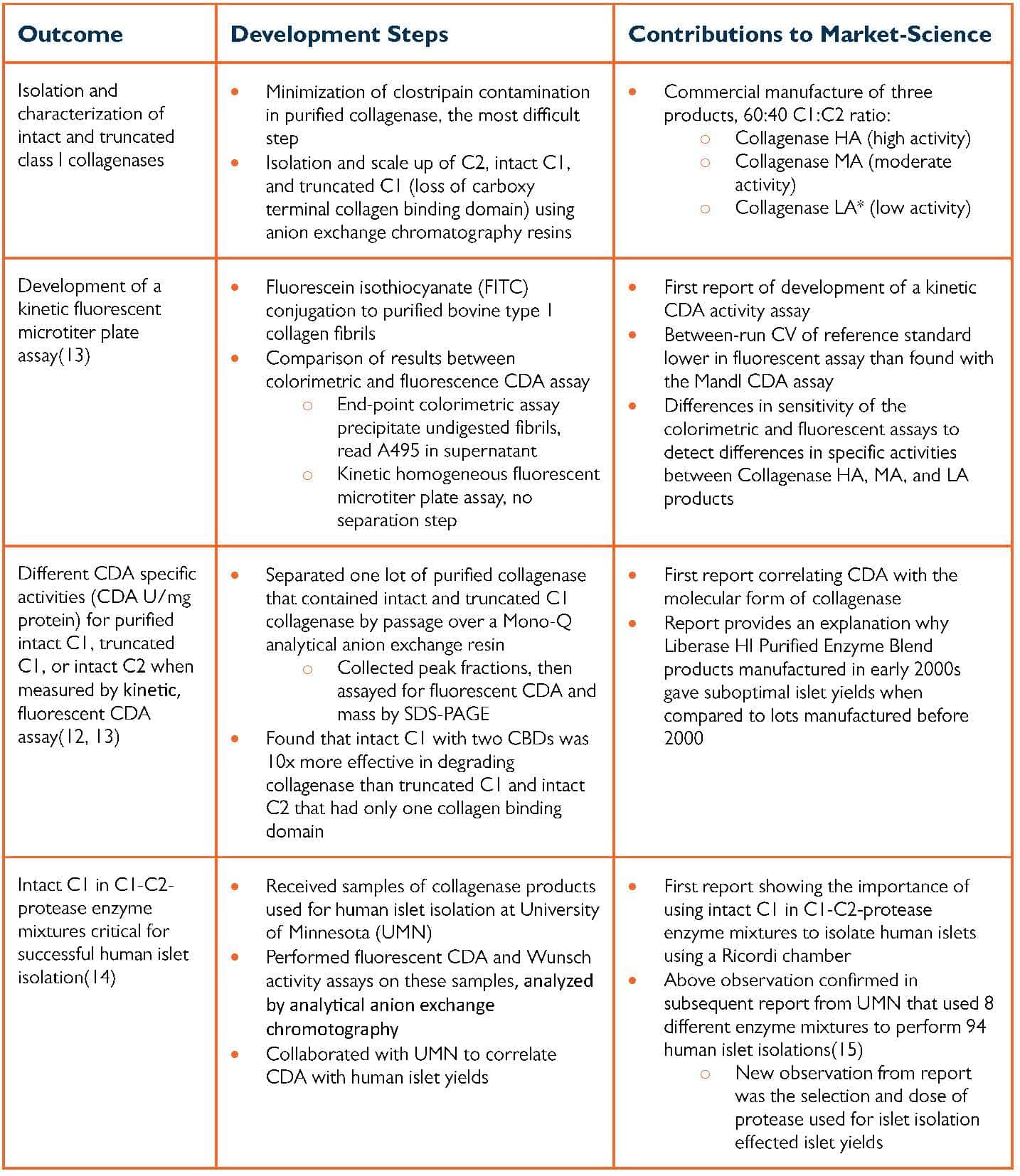
*Collagenase LA dropped, no sales of product
In May, 2009, VitaCyte received a Phase II SBIR award where the goal of the project was to confirm the effectiveness of the recombinant intact C1 (rC1), truncated C1 (trC1) and C2 (rC2) collagenase to recover islets from human pancreas. I modified the last aim of this project from assessing the ability of recombinant collagenase to isolate human islets to determining the optimal dose for rC1 and rC2 on human islet yields. A design of experiment (DOE) approach assessed the impact of collagenase dose on human islet yield. This was the first prospective study to determine the dose of enzyme used for mammalian cell isolation. The outcome from this project is summarized in the table below.
Summary From Phase II SBIR Award
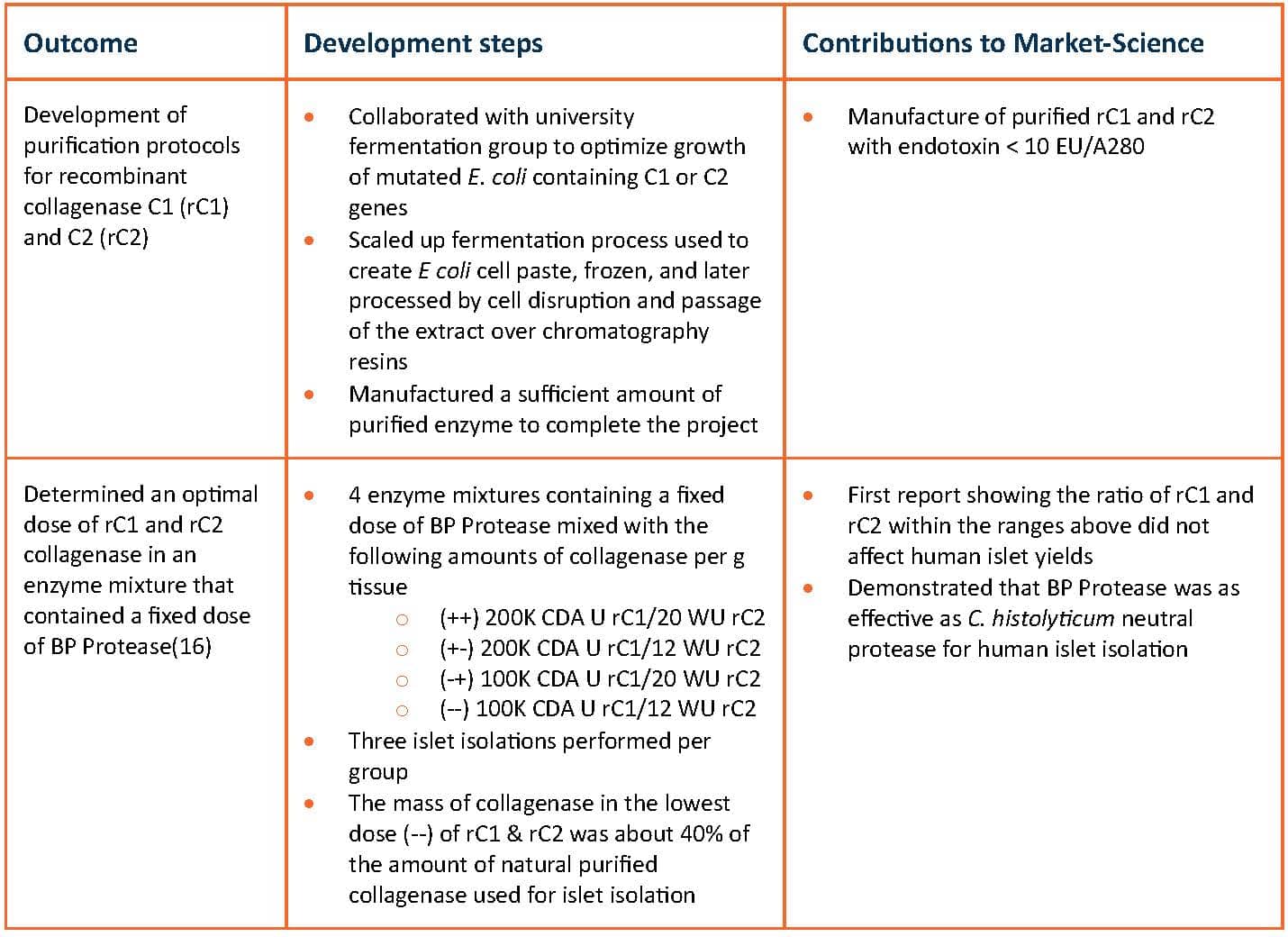
WU = Wunsch Units
The results from this development were clear: rC1 and rC2 collagenases could be used at a lower dose when compared to islets isolated with natural purified collagenases at doses of 20 WU per g tissue. Balamurgan Appakalai’s laboratory at the University of Louisville extended his earlier work at UMN (he managed VitaCyte’s DOE Study) by comparing the effectiveness of the lowest recombinant collagenase dose (100K CDA U rC1, 12 WU rC2, and 23,500 NP U of BP Protease per g tissue) to VitaCyte’s Collagenase HA product used at 20 WU and 23,500 NP U of BP Protease per g tissue.(17) These studies showed that when the dose of natural purified collagenase was reduced to 12 WU per g tissue, the islet yield dropped in half. In contrast, the recombinant collagenase mixture was equally effective at 12 or 20 WU per g tissue.
In 2015, VitaCyte introduced an animal-origin-free (AOF) rCollagenase HI product where the formulation was the midpoint dose from the DOE study described above (15 million CDA U rC1 and 1200 WU rC2 per bottle) for recovering islets from a 100 g pancreas. The University of Alberta evaluated this enzyme mixture and digested human pancreata with 150K CDA U, 16 WU, and 23.5K NP U of BP Protease per g pancreas.(18) The human islet yields for rCollagenase HI were comparable to those found when either the Roche or VitaCyte purified collagenase products supplemented with Thermolysin. However, comparison of the islet parameters from isolations performed with either recombinant or natural collagenase showed that islets isolated with rCollagenase HI gave higher post-purify IPN counts, post-culture IPN, or IE counts, resulting in a significantly higher mass of islets transplanted into the recipients. The other interesting finding from this report was the significantly higher insulin response to glucose challenge by islets isolated with rCollagenase HI-BP Protease enzyme mixtures when compared to those isolated with purified natural collagenase and Thermolysin.
What does the future hold at VitaCyte? We will continue to follow our core belief that correlation of enzymatic and biochemical characteristics to cell yield and function will provide customers with a consistent product. Initially, customers will need to optimize the enzyme dose for their application, but once optimized, the need for lot selection is eliminated. This bold statement is based on the rigorous approach that VitaCyte takes to assess its products by an analytical anion exchange chromatographic analysis and Wunsch activity as described in US Pharmacopeia monograph chapters <89.1> and <89.2>. In addition, routine use of the kinetic fluorescent microtiter plate assay has shown that each functional form of purified collagenase has a distinctive specific activity: intact C1 134,759 CDA U/mg, truncated C1 53,185 CDA U/mg, and intact C2 13,320 CDA U/mg. The combination of these three analyses to assess the structural integrity of C1 and the enzymatic activities of C1 and C2 collagenases, ensures that each lot of product is manufactured more consistently than any other product sold today.
References
- Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl 1992;1:116-26.
- Bond MD, Van Wart HE. Relationship between the individual collagenases of Clostridium histolyticum: evidence for evolution by gene duplication. Biochemistry. 1984;23:3092-9.
- Hatton MW, Berry LR, Krestynski F, Sweeney GD, Regoeczi E. The role of proteolytic enzymes derived from crude bacterial collagenase in the liberation of hepatocytes from rat liver. Identification of two cell-liberating mechanisms. Eur J Biochem. 1983;137:311-8.
- Hefley TJ, Stern PH, Brand JS. Enzymatic isolation of cells from neonatal calvaria using two purified enzymes from Clostridium histolyticum. Exp Cell Res. 1983;149:227-36.
- Ricordi C, Tzakis AG, Carroll PB, Zeng YJ, Rilo HL, Alejandro R, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplant. 1992;53:407-14.
- Dwulet FE, Ellis BB, Gill JF, Jacobsen LB, Smith ME, Waters DG. Purified mixture of collagenase I, collagenase II, and two other proteases. 1998; US Patent 5,753,485.
- McCarthy RC, Green ML, Dwulet FE. Evolution of enzyme requirements for human islet isolation. OBM Transplant. 2018; 2:1-30. Available from: http://www.lidsen.com/journals/transplantation/transplantation-02-04-024.
- Linetsky E, Lehmann R, Li H, Fernandez L, Bottino R, Selvaggi G, et al. Human islet isolation using a new enzyme blend. Transplant Proc. 1997;29:1957-8.
- Olack BJ, Swanson CJ, Howard TK, Mohanakumar T. Improved method for the isolation and purification of human islets of langerhans using Liberase enzyme blend. Hum Immunol. 1999;60:1303-9.
- Lakey JR, Cavanagh TJ, Zieger MA, Wright M. Evaluation of a purified enzyme blend for the recovery and function of canine pancreatic islets. Cell Transplant. 1998;7:365-72.
- Fetterhoff TJ, Cavanagh TJ, Wile KJ, Wright MJ, Dwulet FE, Gill J, et al. Human pancreatic dissociation using a purified enzyme blend. Transplant Proc. 1995;27:3282-3.
- Matsushita O, Okabe A. Clostridial hydrolytic enzymes degrading extracellular components. Toxicon. 2001;39:1769-80.
- Eckhard U, Schönauer E, Nüss D, Brandstetter H. Structure of collagenase G reveals a chew-and-digest mechanism of bacterial collagenolysis. Nature Struct Mol Bio. 2011;18:1109-14.
- Sakon J, McCarthy RC. Collagen degradation by Clostridium histolyticum collagenases. 2021 [Available from: Collagen Degradation by Clostridial histolyticum Collagenases – VitaCyte (wpengine.com).
- McCarthy RC, Breite AG, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: Factors to consider in setting enzyme acceptance criteria. Transplant. 2011;91:137-45.
- McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, Dwulet FE. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplant Proc. 2008;40:339-42.
- Barnett MJ, Zhai X, LeGatt DF, Cheng SB, Shapiro AMJ, Lakey JRT. Quantitative assessment of collagenase blends for human islet isolation. Transplant. 2005;80:723-8.
- Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplant. 2010;89:954-61.
- Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplant. 2012;93:693-702.
- Balamurugan AN, Green ML, Breite AG, Loganathan G, Wilhelm JJ, Tweed B, et al. Identifying Effective Enzyme Activity Targets for Recombinant Class I and Class II Collagenase for Successful Human Islet Isolation. Transplant Direct. 2016;2(1):e54.
- Loganathan G, Subhashree V, Breite AG, Tucker WW, Narayanan S, Dhanasekaran M, et al. Beneficial effect of recombinant rC1rC2 collagenases on human islet function: Efficacy of low-dose enzymes on pancreas digestion and yield. Amer J Transplant. 2018;18:478-85.
- O’Gorman D, Kin T, Rosichuk S, Richer B, Zhai W, Moriarity J, et al. Evaluation of a low dose recombinant collagenase and BP Protease for clinical islet transplantation. International Pancreas and Islet Transplantation Association; 2021: Transplant 105:S20.
