VitaCyteUnravelling Cell Isolation
VitaCyte manufactures and supplies GMP Grade purified-defined Clostridium histolyticum collagenase and protease enzymes to researchers, commercial cell suppliers, and institutions that manufacture cells as therapeutic agents. VitaCyte’s capabilities to custom manufacture products enables collaboration with companies who use enzymes for commercial applications.

Research
Cell Suppliers

Cell Therapy
Our ProductsPurified Cell and Tissue Dissociation Enzymes
Highly Purified
Lot to Lot Consistent
Best Performing Enzymes
Ability to Design Mixtures for Specific Applications
Ability to Customize Product to Meet Your Needs
Purified-Defined EnzymesWhat does it mean?
Collagenase suppliers can be divided into three categories:
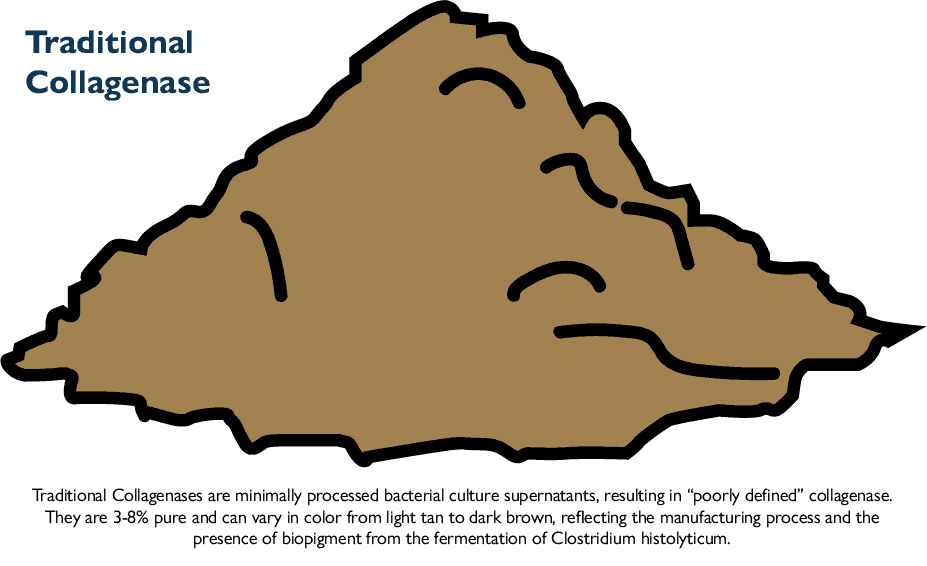
Traditional Crude or Enriched Collagenase
Purified Collagenase
Purified-Defined Collagenase
Our StoryHow VitaCyte Began
VitaCyte’s co-founders, Bob McCarthy and Francis Dwulet, were key contributors to the development of the first commercial, purified collagenase-protease enzyme mixture used to prepare cells for subsequent transplantation. This improved tissue dissociation enzyme reagent contributed to success of the “Edmonton Protocol,” the first report of successful treatment of adult patients with Type 1 Diabetes where 7 of 7 patients were “insulin independent” one year after islet transplantation. Prior to the introduction of this purified enzyme reagent, most laboratories performing this clinical research procedure were spending significant amounts of time qualifying collagenase lots for their effectiveness to isolate human islets. Once a lot was selected, the lot qualification procedure was repeated within a year, often because of the instability of the enzymes on storage.
ResourcesAll things cell isolation
Overcoming the Challenges of Islet Recovery from Young Donor Pancreases: An Update 5 Years Later
Thursday, March 12, 2026 | 11 AM ESTPresented by Appakalai N. Balamurugan, PhD My laboratory described a novel approach to this problem by comparing the effect of different collagenase-protease enzyme mixtures on islet yields from young donors. For these islet...
[CTRMS 2025] ISO 20399 Standard for Ancillary Materials: Relevance to Tissue Dissociation Enzymes
VitaCyte is sponsoring a Symposia. ISO 20399 Standard for Ancillary Materials:Relevance to Tissue Dissociation Enzymes Robert McCarthy Thursday, October 23 | 12:30-13:30 Two published Standards on ancillary materials (AMs) are the US Pharmacopeia (USP)...
The ISO 20399:2022 Ancillary Material Standard Emphasizes the Need for Renewed Focus on Tissue Dissociation Enzymes Used for Human Islet Isolation
Robert McCarthy Author’s note: I will make a similar presentation to the one cited below as part of VitaCyte’s webinar series at 11 AM EST on Thursday, December 11, 2025 Importance of Controlling Critical Ancillary Materials Ancillary materials (AMs) come in...
For questions regarding enzyme useage, regardless if they are directly related to VitaCyte’s products, Customer Service is excellent. People at the company are eager to share their valuable experience and opinions on how best to perform procedures from donors of various species.
Latest NewsAll things cell isolation
SOT 2026
Society of Analytical Toxicology 65th Annual Meeting San Diego, California March 22-25, 2026AttendingBob McCarthy and Drew Breite will be attending Society of Analytical Toxicology 65th Annual Meeting in San Diego, California from March 22-25, 2026. Look for them at...
Employee Spotlight
January - March 2026 We like to celebrate every milestone of our employees who make VitaCyte possible.happy birthdayDan CollinsDrew BreiteDonna Skinnerhappy work anniversaryMike Green18 years | Quality ControlAmber McDonough8 years | FinanceDan Collins12 years |...
EPITA 2026
15th EPITA Symposium 2026 Innsbruck-Igls, AustriaJanuary 25-27, 2026AttendingBob McCarthy and Peter Frost will be attending and exhibiting at EPITA 2026 in Innsbruck-Igls, Austria from January 25-27, 2026. Both will be available to discuss technical questions related...



![[CTRMS 2025] ISO 20399 Standard for Ancillary Materials: Relevance to Tissue Dissociation Enzymes](https://www.vitacyte.com/wp-content/uploads/2025/09/CTRMS-2025-400x250.png)



