Rodent Hepatocyte ApplicationsRecommended Products
Use a collagenase and a protease to supplement for rodent hepatocyte applications.
Modified Seglen’s Two-Step Isolation Method (1,2)Recommended Method
Note: Tissue digestion is stopped when cells under Gilson’s capsule (membrane that encapsulates the liver) move freely in the tissue. The timing is determined by the expertise of the individual performing the isolation.
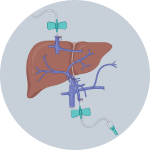
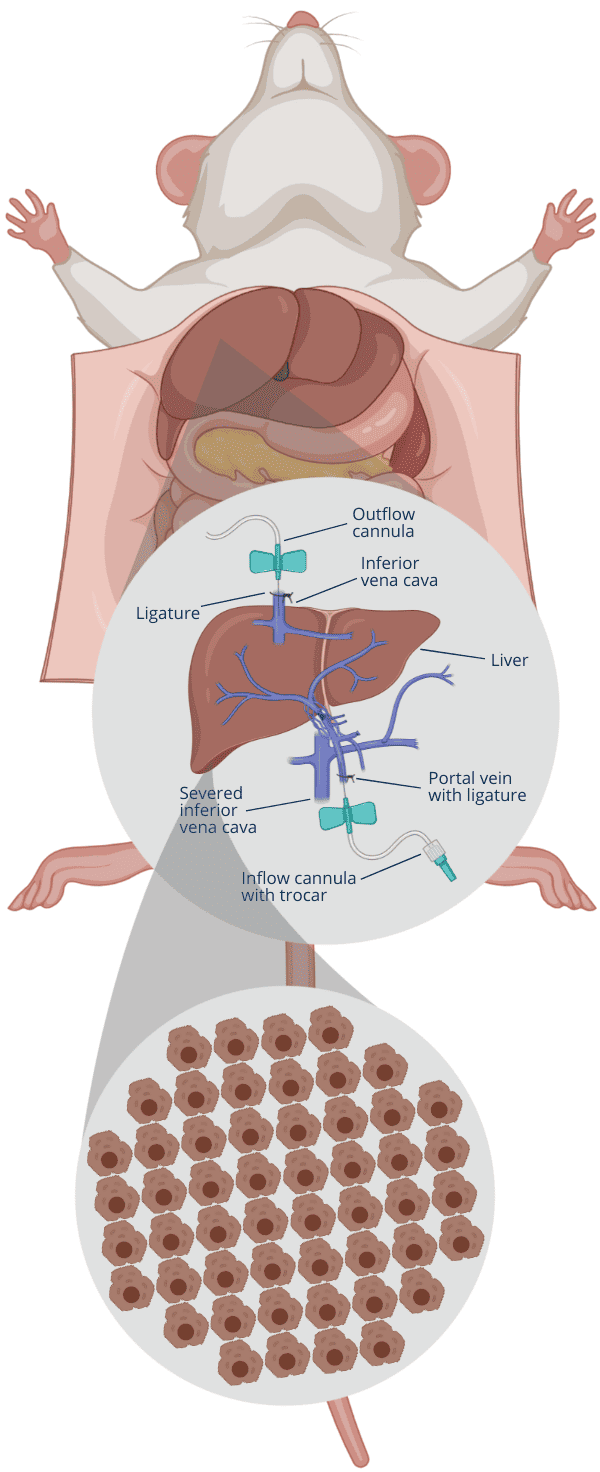
1Insert cannulas into portal vein and inferior vena cava

2Flush blood out by infusing Solution 1 via portal vein
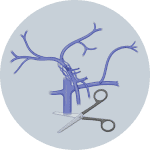
3Cut inferior vena cava when Solution 1 enters the liver

4After Solution 1, infuse Solution 2 via portal vein

5After 5-10 min flush, infuse Solution 3 to digest tissue
6Stop digest based on your criteria, cut liver, disperse cells
7Filter cells through mesh screen using Solution 4
8Sediment cells by centrifugation, wash 2x with Solution 4

9Evalutate the cell count and viability by microscope
10Place cells on tissue culture dish for later downstream use
Solution 1
Loosen desmosomes
Calcium Magnesium Free Hank’s Balanced Salt Solution (HBSS), EDTA
Solution 2
Restore Calcium Magnesium & ensure enzyme activity is not inhibited HBSSSolution 3
Loosen cells from organ Collagenase MA (Cat. # 001-2030) 2500 CDA Units per mL BP Protease (Cat. # 003-1000) 1000 Neutral Protease Units per mLSolution 4
Wash cells HBSSSource: Berry, Michael N., et al. Laboratory Techniques in Biochemistry and Molecular Biology. Isolated Hepatocytes: Preparation, Properties and Applications. Vol. 21, Elsevier, 1991.
Find the protocol infographic here. This protocol illustration is created with BioRender.com.
Expected Cell Yield
For many laboratories, recovery a sufficient number of hepatocytes is not an issue since the liver is the largest internal organ in mammals and makes up 4-6% of the body weight of the rodents.(3) A 150-300 g hooded Wistar rat has 109 cells with about 60-70% being hepatocytes. Typical rat liver cell yields are between 30-60 million cells per g of tissue but these yields can be affected by the nutritional state of the animal.(5)
Perspectives
Seglen’s two step procedure was a modification of a procedure initially developed by Berry & Friend in 1969.(4, 5) Seglen’s procedure overcame the variability of the earlier procedure since EDTA or EGTA chelation of calcium ensured that the tight junctions (i.e., desmosomes) between hepatocytes were disrupted in the first step of the procedure. In the second step, collagenase- protease enzyme mixtures in Hanks BSS were perfused throughout the tissue, leading to the recovery of most cells from tissue. It is essential that the collagenase solution is supplemented with calcium to maintain its enzyme activity.(1, 5)
Crude collagenase was and still is used as the primary reagent for most rodent hepatocyte isolations. Both Seglen and Berry have commented that variability in yield and quality of hepatocytes is due to the lot to lot variability of this reagent.(1, 5) We have shown the variability in biochemical composition and performance of different lots of Sigma Type XI collagenase in isolating human hepatocytes.(6)
Resources & Reviews
The Seglen procedure is commonly used today to isolate hepatocytes from many different species. Additional modifications were made to meet the needs of different users. A superb methods book by Berry and colleagues summarizes the isolation and application of hepatocytes as of 1991.(5) The European Center for Validation of Alternative Methods (ECVAM) held a workshop to summarize a list of recommendations for rat hepatocyte isolation.(7) JoVE and other support material have also been published to describe rat(8) and mouse(9) hepatocyte isolation procedures. Recently, a protocol for isolating rodent hepatocytes using VitaCyte’s enzymes was published in Protocols.io.(10)
Open Science Link
https://www.protocols.io/view/rodent-liver-2-step-collagenase-perfusion-and-dige-q26g7p74qgwz/v1
References
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biology. 1976;13:29-83.
- Seglen PO. Isolation of hepatocytes. In: Celis JE, editor. Cell biology : a Laboratory Handbook. 1. San Diego :: Academic Press; 1994. p. 96-102.
- Arms AD, C.C.Travis. Reference physiological parameters in pharmacokinetic modeling. Washington DC: Environmental Protection Agency; 1988.
- Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biology. 1969;43(3):506-20.
- Berry MN, Edwards AM, Barritt GJ. Isolated hepatocytes: preparation, properties and applications. New York: Elsevier; 1991.
- Gramignoli R, Green M, Tahan V, Dorko K, Skvorak K, Marongiu F, et al. Development and application of purified tissue dissociation enzyme mixtures for human hepatocyte isolation. Cell Transplant. 2012;21:1245-60.
- Blaauboer B, Boobis A, Castell J, Coecke S, Groothuis M, Guillouzo A, et al. Practical applicability of hepatocyte cultures in routine testing: the report and recommendations of ECVAM Workshop 1. Alternatives to laboratory animals: ATLA. 1994;22:231-41.
- Shen L, Hillebrand A, Wang DQ, Liu M. Isolation and primary culture of rat hepatic cells. J Visualized Experiments : JoVE. 2012 29(64).
- Feng M, Divall S, Wu S. An Improved Time- and Labor- Efficient Protocol for Mouse Primary Hepatocyte Isolation. Journal of visualized experiments : JoVE. 2021 Oct 25(176).
- Sai.Chung, Sekhon M, Ma X-Z, Manuel J, Cheng ML, MacParland S, McGilvray I. Rodent Liver 2-Step Collagenase Perfusion and Digestion using VitaCyte: Protocols.io; 2024.