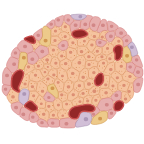
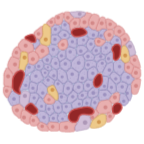
Rodent Islet Applications
Recommended Product
Recommended Method: Gotoh Method1
Use appropriate dose of a collagenase containing enzyme solution injected in situ through the common bile duct that is connected to the main pancreatic duct. The injection of this fluid swells the pancreas, resulting in additional mechanical damage to the tissue structure. The pancreas is removed from the animal, trimmed free of fat, then incubated in an appropriate vessel at 37° for a predetermined amount of time. At the end of the incubation period, the tube is vigorously shaken for less than 30 seconds to release the islet from the tissue. Islets are separated from the acinar cells by density gradient purification, handpicking, or by using both methods: picking islets from an enriched islet preparation.
Protocol for including CIzyme RI in the Gotoh method is found in the CIzyme RI package insert in the Product Documentation page.
Expected Cell Yield
Cell yield varies by sex, age, strain, and nutritional state of the animal. Consult VitaCyte’s white paper entitled “What is an acceptable rodent islet yield?” that provides information from each strain on the average islet yield per pancreas, method used, product and dose used in isolation, digest time, and purification method.
Perspectives
A key component of a successful diabetes research program is consistent and cost effective isolation of high quality rodent islets. The two common methods used to isolate rodent islets are based on the procedure initially described by Lacy & Kosatianovsky2 (Lacy procedure), later modified significantly by Gotoh, et al1 (Gotoh procedure). Both procedures inject Hanks Balanced Salt Solution (HBSS) into the pancreas through the common bile duct that is connected to the main pancreatic duct. The injection of this fluid leads to a swollen pancreas. The primary difference in the procedures is summarized in the table below.
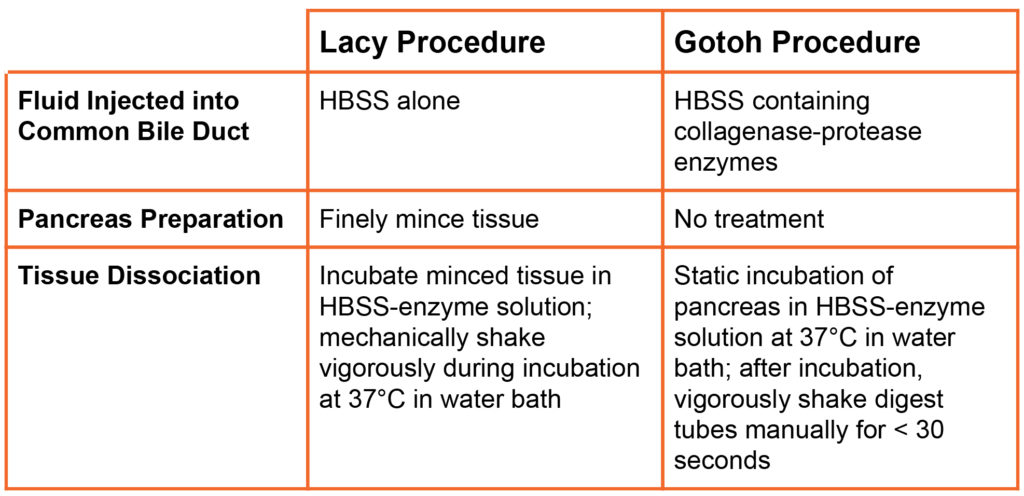
Gotoh’s modification to the Lacy procedure simplifies the digestion of the pancreas by eliminating the need for mincing tissue and continual shaking of the tissue digest during the incubation period. It also avoids the requirement to pretreat all glassware used in the procedure with silicone prior to use. Using either procedure, islets are separated from the acinar cells by density gradient purification, handpicking, or by using both methods: picking islets from an enriched islet preparation.
Resources & Reviews
Four method videos reviewing the Gotoh method are published in the Journal of Visualized Experiments (JoVE) that review key aspects of the method and provide useful technical tips3-6.
Separate reviews describe the Lacy7 and Gotoh8 procedures in more detail.
References
- Lacy PE, Kostianovsky M. Method for the isolation of intact Islets of Langerhans from the rat pancreas. Diabetes 1967; 16: 35-9.
Gotoh M, Maki T, Satomi S, et al. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation 1987; 43(5): 725-30.
Szot GL, Koudria P, Bluestone JA. Murine pancreatic islet isolation. Journal of visualized experiments : JoVE 2007; (7): 255.
Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. Journal of visualized experiments : JoVE 2011; (50).
Neuman JC, Truchan NA, Joseph JW, Kimple ME. A method for mouse pancreatic islet isolation and intracellular cAMP determination. Journal of visualized experiments : JoVE 2014; (88): e50374.
Kelly CB, Blair LA, Corbett JA, Scarim AL. Isolation of islets of Langerhans from rodent pancreas. Methods in molecular medicine 2003; 83: 3-14.
Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biological procedures online 2009; 11: 3-31.