General
Yes.
The supplemental protease used for cell isolation is determined by the precedent set by the enzyme suppliers. However, it is likely that other proteases will also work but it needs to be determined by experimentation. Three different proteases have been used to isolate human islets: thermolysin, BP Protease (a Dispase equivalent enzyme), and C. histolyticum neutral protease. Substitution of one protease for another may not be a seamless transition, however, as the kinetics of digestion can be dramatically different among the various general proteases. For guidance in selecting a protease, contact VitaCyte’s technical support team.
Yes and No.
The most effective inhibitor of protease activity is to use serum-containing media. Adult serum is more effective than fetal serum. The protease activity is likely inhibited by alpha-2-macroglobulin. Buffers or media containing human serum albumin (HSA) are ineffective in suppressing protease activity, even at very high concentrations of protein (>10%).
Collagenase activity is not inhibited by serum-containing media/buffers. The most effective method to reduce collagenase activity is to reduce temperatures. Collagenases will lose about 80% of its maximal activity at 26°C. The use of an ice-cold, serum-containing media should eliminate all the proteolytic activity and reduce the collagenase activity in the enzyme containing solution.
No and yes.
For poorly defined collagenase products, many isolators use these values as a guideline in selecting a specific lot of collagenase. These analyses are an unreliable predictor of successful cell isolation because the collagenase is often degraded by proteases during the manufacturing or manufacturing procedure. For defined collagenase products, if the manufacturer claims or shows data that the collagenase is intact and the protease activity is tightly controlled, then performing the appropriate enzymatic analysis can be a useful tool to predict the success of islet isolation.
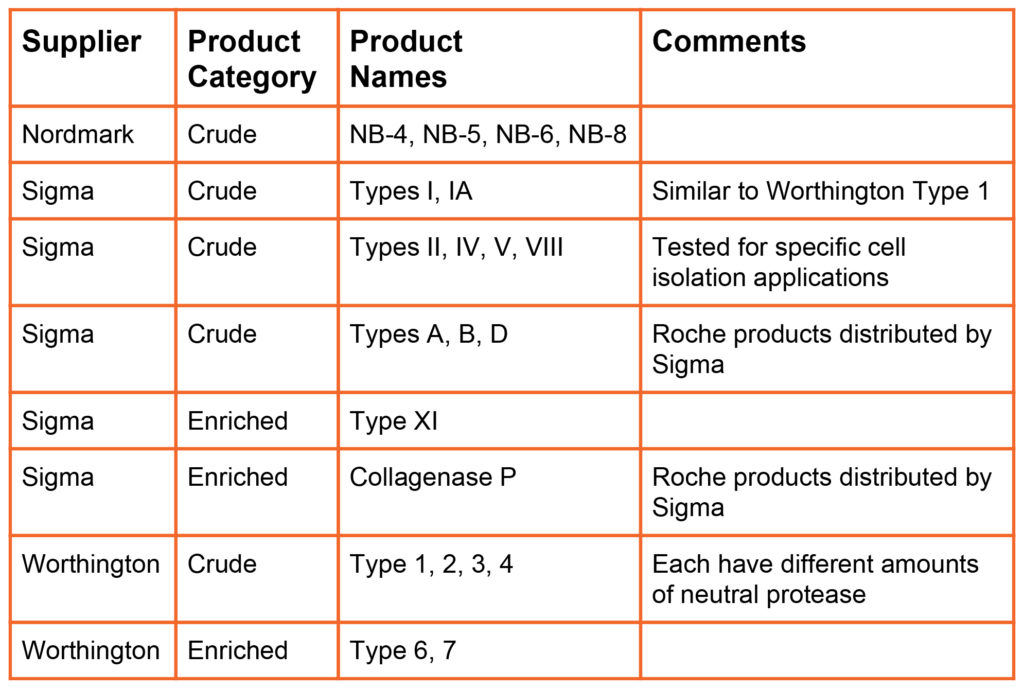
Initially, crude collagenase manufactured and sold by Worthington Biochemicals in the early 1960’s and later by Sigma-Aldrich. These products are minimally processed bacterial culture supernatants. Later, Sigma manufactured Collagenase Type XI, an enriched collagenase product prepared by further processing of crude collagenase. Enriched collagenase has higher enzyme activities than found in crude collagenase. All these products can be classified as “poorly defined” collagenase because the biochemical composition reflects the composition of the bacterial culture supernatant. Each lot of product is unique. No attempt is made to dramatically alter the enzyme composition, Collagenase represents 3-8 and 10-20% of the dry weight of crude and enriched collagenase, respectively.
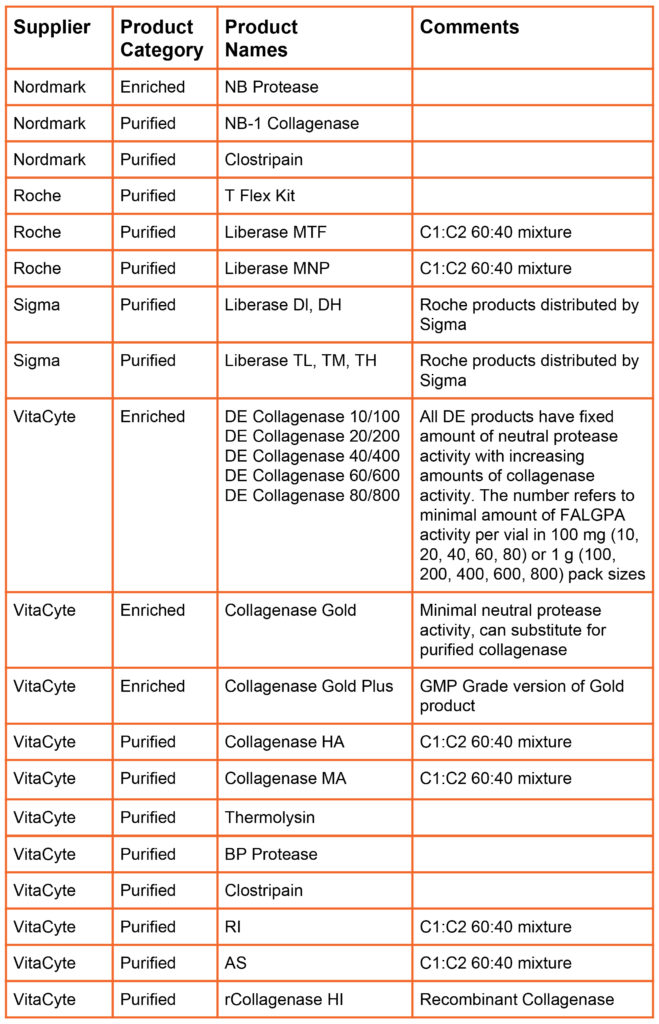
In 1994, Boehringer Mannheim Biochemicals introduced the Liberase Purified Enzyme Blend product line. The Liberase products were purified enzyme mixtures containing class I (C1) and class II (C2) collagenase and a neutral protease. The Liberase products created a new product category: “defined collagenase.” with Liberase categorized as a purified, defined collagenase enzyme mixture. These products are more expensive than poorly defined crude or enriched collagenase products because collagenase had to separated from other proteins in the culture supernatant, then class I and class II collagenase was separately purified, then recombined in the final product.
In 2015, VitaCyte introduced an enriched, defined collagenase products for research use where the collagenase is > 85% pure and consists primarily of undegraded enzyme. DE Collagenase, Collagenase Gold that has characteristics similar to the Liberase products at a much lower cost since there was no need to perform downstream purification of the enzyme. These products are priced similarly to crude or enriched collagenase products.
The table below summarizes the products offered by different primary collagenase suppliers (i.e., suppliers that manufacture and sell collagenase products). Nordmark and VitaCyte offer animal origin free versions of purified collagenase and neutral protease products. Nordmark, Roche, and VitaCyte offer GMP Grade versions of many of their products.
Poorly Defined Products
Defined Products
C. histolyticum collagenase and many other bacterial neutral proteases are metallo-proteases. They require excess calcium to maintain their enzyme activity. One mM calcium ion is sufficient to protect this activity.
Cation chelating agents such as EDTA or EGTA should not be used in the presence of these enzymes. As many of you know, these chelating agents are routinely used in the isolation of mammalian hepatocytes using Seglen’s two step procedure. However, the solutions containing chelating agents are used in a separate step of this procedure and are washed out of the tissue before perfusion of the collagenase solution in a calcium containing buffered media.
Phosphate buffered saline enzyme solution can be used for cell isolation but prolonged or frozen storage of the enzyme in this solution will lead to loss of collagenase or protease activity. Phosphate chelates calcium, leading to loss of enzyme activity.
Nearly all collagenases used for cell isolation are derived from culture supernatants recovered after anaerobic culture of Clostridium histolyticum. A rich broth containing digested animal meat is frequently used to ensure growth of C. histolyticum. C. histolyticum is a saprophyte that survives by secreting a diverse number of enzymes that breaks down decaying organic matter. These enzyme activities include proteases, glycosidases, lipases, and nucleases.
The initial fermentation procedures to generate collagenase or other bacterial enzymes required the use of mammalian meat peptones that contained collagen peptides. As collagenase fermentation methods were refined, gelatin peptones were used in place of meat peptones. You can request a certificate of origin from the manufacturer to determine if animal proteins were used in the fermentation or manufacturing process.
Products labeled AOF means no animal products were used in the fermentation procedure to generate collagenase or neutral protease. The absence of animal products in the manufacturing procedure avoids providing additional explanations to regulatory authorities on the potential threat of an adventitious agent contaminating the product.
Each lot of poorly defined crude or enriched collagenase is unique because it represents the enzymatic activities present in the C. histolyticum culture supernatant. The enzyme activities listed on the Certificate of Analysis can be used as a guide but they are not sufficient to predict the effectiveness of a specific lot to recover cells. Lot pre-qualification enables the user to evaluate the performance of the new lot against the collagenase lot that is currently in use. If the new lots do not give acceptable results, then additional lots are evaluated. Once an acceptable or “good” lot is identified, then the cell isolator purchases an amount of product sufficient to perform > 1 year of cell isolations.
Enzyme Activity
No.
Although each manufacturer may use similar methods, they will use different lots of substrate from different suppliers, make additional modifications to the assay procedure, as well as other changes, it is safe to assume there is poor correlation when comparing enzyme activity results from different suppliers.
No.
Poorly defined, traditional collagenase products contain between 3-8 and 10-20% (w/w) collagenase. Collagenase is usually degraded during the fermentation or material processing procedure. Recent studies have indicated that proteolytic degradation of collagenase leads to lower collagen degradation activity that translates into use of higher amounts of enzyme in the cell isolation procedure.
There is better correlation of collagen degradation activity to cell yields when a consistently manufactured collagenase with minimal proteolytic damage of collagenase is used in the cell isolation procedure. However, no rigorously controlled studies have studied this issue. The reason for this observation can be explained by the model of enzymatic tissue dissociation described below.
Clostripain is a neutral protease secreted by C. histolyticum during the fermentation process. It is a tryptic like enzyme that is measured using the same peptide substrate to measure trypsin activity (N-benzoyl-L-arginine ethyl ester, BAEE). Clostripain is a sulfhydrl enzyme that expresses maximal enzyme activity under reducing conditions (i.e., pre-incubation with 2 mercapatoethanol or other reducing agent). It is reported on a Certificate of Analysis as clostripain or total clostripain activity. Trypsin like activity measures clostripain activity in the absence of reducing agent. It usually represents about 10% of the total clostripain activity.
Collagenase activity can be assessed by a number of different enzyme activity assays. The enzyme activity is defined by the substrate so
- peptidase activity is detected when Pz-peptide (Wunsch assay) or FALGPA is used as substrates;
- gelatinase activity is detected when gelatin or azocoll are as substrates;
- collagen degradation activity (CDA) is detected when using collagen fibers (Mandl assay) or FITC collagen fibrils (kinetic fluorescent microplate CDA assay) as substrate.
C. histolyticum class II collagenase has about a 50 fold higher peptidase activity than a comparable amount of class I collagenase. Similarly, class I collagenase has about a 2-5 fold higher gelatinase activity than class II collagenase. This difference in enzyme activity profiles led to definition of class I and class II collagenase.
Recently it has been shown that the CDA activities are different depending on the assay and substrate used to detect this activity. Worthington and Sigma use the Mandl CDA assay to detect this activity. Intact or truncated class I collagenase has about a 2-3 fold higher specific activity than intact class II collagenase. In contrast, in the kinetic fluorescent microplate CDA, the activity correlates with the number of collagen binding domains on collagenase. Intact class I collagenase with two collagen binding domains has 7 to 10 fold higher specific CDA (U/mg protein) than intact class II or truncated class I collagenase, each having one collagen binding domain.
Mechanism
Maybe.
It is very difficult to do for several reasons. First, the assays reported on the Certificate of Analysis do not provide sufficient detail to define the different functional forms of collagenase. Collagenase in crude or enriched collagenase products contain intact class I and class II, truncated class I collagenase and degraded forms of class I or class II collagenase that cannot degrade native collagenase (non-functional forms). The Mandl assay is commonly used to assess collagen degradation activity in traditional collagenase products. Recently, it has been shown that this assay can be imprecise and the collagen degradation activity reflects the class or collagenase rather than its efficiency to degrade native collagen.
The Liberase projects showed that collagenase and neutral protease were the enzymes required for the release of islets from human pancreatic tissue. Liberase contains purified class I and class II C histolyticum collagenase, and thermolysin or Dispase. Different formulations and doses were used to isolate cells from different tissues.
The identification of the C. histolyticum genes and corresponding protein domain structures of class I and class II collagenase provided new insights into the mechanism of enzyme-mediated tissue dissociation. Both classes of collagenase contain four protein domains: a large catalytic domain that cuts native collagen or gelatin, linking domain(s) (no known function), and collagen binding domain(s). Intact class I has one catalytic domain, a linking domain, and two collagen binding domains whereas intact class II has a catalytic domain, two linking domains, and one collagen binding domain. Only those forms of collagenase containing a catalytic domain and at least one collagen binding domain can degrade native collagen (collagen fibrils or fibers). Collagenase must bind via a collagen binding domain(s) to native collagen for the catalytic domain on the same molecule to digest the collagen monomer (i.e., tropocollagen). A functional catalytic domain, with or without a collagen binding domain, express gelatinase or peptidase (FALGPA or PZ peptide) activity.
Class I collagenase binds native collagen in the extracellular matrix to initiate the collagen degradation process. The collagen binding domain(s) then “pulls” the catalytic domain from the carboxy to amino terminal end of a collagen monomer within a collagen fibril or fiber, digesting tropocollagen. Class II is required for effective collagen degradation but the mechanism is not defined.
As collagenase cuts native collagen, the tight, protease resistant structure of the extracellular matrix is relaxed leading to exposure of protease sensitive sites on other extracellular matrix proteins. When a sufficient number of these proteins that anchor cells to the matrix are cut, then cells are released from the tissue.
Only three forms of C. histolyticum collagenase can degrade collagen. The forms are intact class I, intact class II and “truncated” class I where the carboxy terminal collagen binding domain is lost by proteolysis. Intact class I has one catalytic and two collagen binding domains whereas the other two forms have one catalytic domain and one collagen binding domain.
The success of cell isolation requires excess collagen degradation activity and selection of an appropriate neutral protease used at an optimal dose. This statement reflects the two assumptions of enzymatic tissue dissociation:
- Enriched or purified collagenase (with minimal contaminants) has a restricted specificity to degrade only native collagen or gelatin which are extracellular matrix proteins. Excess activity will not impact the degradation of collagen nor damage cells.
- Neutral protease is required to degrade the extracellular matrix proteins. Proteases can also damage proteins on the cell membrane. Therefore, choice of the appropriate protease used at the right dose is critical to success.
Products
To be determined.
VitaCyte’s products are labeled for research use only or as GMP Grade. All products are manufactured in accordance with the principles for clinical trial material outlined in the International Conference on Harmonization (ICH) document ICH Q7A, “Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients”. The GMP Grade products are bottled and lyophilized under more tightly controlled conditions. Our internal document control system is in alignment with FDA guidance for Phase I material as described in 21 CFR Parts 210 and 211. In addition, document controls are in place to minimize the chances of cross-contamination. Guidance for use of reagents in clinical cell transplantation procedures is governed by local Institutional Review Boards and regional Health Authorities. Presently, the FDA or EMA have not defined any requirements for use of enzymes as ancillary products in therapeutic cell isolation.
No.
VitaCyte’s purified collagenases and neutral proteases, as well as our application specific formulations, are all aseptically dispensed in a biological safety cabinet located within a controlled environment. To ensure sterility, we recommend the user filter the enzyme solution through a 0.2 µm sterile filter immediately before use.
Yes.
Internal studies have shown the reconstituted collagenase is stable as a frozen solution between -15 to -25°C for at least 1 year as long as no other protease enzymes have been added to the collagenase solution. For ensured stability it is best to minimize the volume used for dissolution and reconstitute with water or a phosphate-free buffer. Additional studies have shown the reconstituted collagenase was successfully frozen and thawed three times as a concentrated solution with no apparent loss of potency as assessed by the CDA assay.
It is difficult to come up with a reliable conversion factors to translate enzyme activities from one supplier to those from another supplier. Some of the factors that may affect this value are differences in the assay precision by one or both suppliers, differences in the protein or derivatized substrates to detect the activity of a specific protease, and the method used to detect protein degradation.
Any conversion factor used to translate enzyme activities between should be used as a guideline and not as an absolute value. For additional information, contact VitaCyte technical service.
Yes.
VitaCyte’s collagenase products are chromatographically purified to minimize contamination of protease. Collagenase with negligible protease contamination ensures minimal loss of collagen degradation activity during storage or as a frozen solution. Purified collagenase alone is ineffective in releasing cells from tissue since protease activity is required to degrade other extracellular matrix proteins (see Mechanism FAQs). Therefore, the selection of an appropriate protease used at an optimal dose is likely the most important decision a cell isolator can make to ensure recovery of an adequate amount of functional cells.
Product abbreviations:
- Collagenase HA = purified collagenase with high collagen degradation activity (CDA)
- Collagenase MA = purified collagenase with moderate CDA (about 50% of Collagenase HA)
- BP Protease = neutral protease from Bacillus polymyxa (DispaseTM Equivalent enzyme)
- DE Collagenase = Defined Enriched collagenase
- CIzyme RI = application specific enzyme mixture for rodent islet isolation
- CIzyme AS = application specific enzyme mixture for isolation of human adipose stromal vascular fraction cells from lipoaspirate
Abbreviations in technical literature:
- C1 = class I C. histolyticum collagenase
- C2 = class II C. histolyticum collagenase
- Truncated C1 = form of C1 collagenase with only one collagen binding domain (other lost by proteolysis)
To convert DMC units to FITC-BSA units, multiply the DMC units by 13,400. For example, if you are currently using 1.75 DMC U per gram then you would target 23,450 FITC-BSA units for equivalent activities.
The DispaseTM II product is a cruder preparation of neutral protease than our CIzyme™ BP protease, but both originate from Paenibacillus polymyxa fermentations . The FITC-BSA specific activity of the Dispase II product is less than 10% that of our CIzyme™ BP Protease and can vary dramatically between lots. To substitute CIzyme™ BP Protease for Dispase II, a good rule of thumb is to replace Dispase II targeted at 1.0 mg/mL with 5,000 FITC-BSA Units/mL (roughly 30 µg/mL CIzyme™ BP Protease).
Refer to the package insert for each product. If you have further questions, contact VitaCyte technical support.
Refer to the package insert for each product. If you have further questions, contact VitaCyte technical support.
This is dependent on the cell you are isolating.
VitaCyte has enzyme product/formulations and recommended doses to isolate:
- Rodent Islets
- Porcine Islets
- Non-Human Primate Islets
- Human Islets
- Rodent and Human Hepatocytes
- Rodent Cardiomyocytes
- Human Adipose
These recommendations can be found on the Applications page.
For other cells, view the other cells page that translates the concentrations of different supplier products into recommended products and doses of the DE Collagenase products.
You can also contact VitaCyte’s technical service team that has a significant amount of experience working with customers to have them transition switching enzymes in their cell isolation procedures. The advantage of using VitaCyte’s product in place of traditional collagenase products is that once product and dose of enzyme used in an isolation is determined, there is no need to perform lot qualification again. VitaCyte’s products are consistently manufactures and the neutral protease activity, the key variable in any enzyme formulation, is tightly controlled to minimize variability between lots.
The DE Collagenase, Collagenase Gold, Collagenase Gold Plus, BP Protease, and CIzyme AS products are stable for several days at ambient temperatures. For other products, contact VitaCyte technical support.
Application Specific Formulations are pre-blended mixtures of purified collagenase and protease in optimal ratios for isolation of cells from a particular tissue and/or species. These formulations provide a recommended dose for use with an associated method. These effectiveness of these product to isolate specific cell types was demonstrated by several different laboratories. Currently, VitaCyte offer 3 application specific product to isolate rodent islets (CIzyme RI), human adipose stromal vascular fraction cells (CIzyme AS), and human islets (rCollagenase HI).
Temperatures in the range of 35-37°C will result in maximum activity of collagenase degradation of native collagen. A 5°C reduction in temperature (30°C) will result in an approximate 60% loss of maximal activity for class 1 and class 2 collagenase. In contrast, the neutral proteases BP Protease and thermolysin are less dramatically affected by temperature and maintain ≥ 80% their maximal activity at 26°C.
We recommend using sterile or RODI water for the initial reconstitution of the lyophilized protein. For details, refer to the products’ package insert. After dissolution, dilute the enzyme to the appropriate volume for use in your application. If you want to use a solution other than water to reconstitute the lyophilized protein, avoid using solutions that contain chemicals or chelating agents that can bind to metal cations (e.g., zinc, calcium). These cations are critical for maintaining the enzyme activity of bacterial collagenase or neutral protease (e.g., BP Protease, Dispase, Thermolysin, clostripain, C. histolyticum neutral protease). Any material that contains a metal chelating agent (e.g., EGTA, EDTA) should never be used. The phosphate in phosphate buffered saline will remove calcium from collagenase if stored for long periods of time so it is strongly recommended not to store bacterial collagenase or protease in this solution. If using a culture media such as CMRL 1066 or RPMI 1640, avoid serum as it will inhibit protease activity.
CIzyme Collagenase HA (high activity) and MA (moderate activity) differ only in the amount of truncated class 1 (C1) collagenase present. Truncated C1 is defined as that form of C1 that has lost the carboxy terminal collagen binding domain. Truncated C1 has a mass about 90% of intact C1. Both HA and MA are 60%:40% blends of highly purified class 1 and class 2 collagenase (C2), respectively. Collagenase MA will have a lower CDA specific activity relative to the HA product, because it contains intact and truncated C1. By contrast, Collagenase HA contain predominantly intact C1. (i.e., C2 and truncated C1). The two products have comparable Wunsch specific activities.
Refer to the package insert for each product. If you have further questions, contact VitaCyte technical support.
There are over 200 mature cell types in mammals. A limited number of these cells are routinely used for research studies. These include islets and acinar cells from the pancreas, hepatocytes and non-parenchymal cells from the liver, cardiomyocytes, neural cells, and different types of kidney cells. For many of these cells, VitaCyte provides a product, reference to a method, and dose recommendation.
For other types of cells, it is difficult to make a recommendation because of the use of traditional collagenase product in the procedure where each lot of product is unique. There are also differences in the cell isolation methodology performed in different laboratories. In these cases, review the other cells web page that translates the concentrations of different supplier products into recommended products and doses of the DE Collagenase products (see other cells).
If you want to consider working collaboratively to optimize the enzyme composition and dose used to isolate a specific cell type, contact VitaCyte technical support.
These results cannot be compared since different substrates and assay principles are used to measure this activity. VitaCyte’s kinetic fluorescent microplate CDA assay detects an increase in fluorescence as collagenase unravels and degrades fluorescein isothiocyanate conjugated to collagen fibrils. Measurements are made every 150 seconds.
By contrast, the Mandl is an endpoint assay where collagenase is incubated for 5 hours with collagen fibers. The assay tubes are centrfuged and a portion of the supernatant is assayed for free amino acids and small peptides using a reagent to detect free amine groups. After a two hour incubation, the results are compared to a standard curve using purified leucine. One enzyme unit is equivalent to the presence of one micromole of leucine per minute in the supernatant.