
Robert McCarthy
Paradigm is a useful but often overused concept. Thomas Kuhn introduced the term paradigm in his 1963 book, The Structure of Scientific Revolutions.(1) The Oxford English dictionary defines paradigm as a philosophical worldview that “underlies the theories and methodology of science in a particular period of history.” A paradigm shift is a “major conceptual or methodological change in the theory or practice of a particular science or discipline.”(2) New paradigms arise when the prevailing paradigm cannot or poorly explain an established worldview. The generation of new paradigms is often driven by a new perspective or the use of new methods/tools that provide new insights that underlie the development of a new paradigm.
Joel Barker’s 1993 book, entitled Paradigms, the Business of Discovering the Future, extended Kuhn’s paradigm concept by defining it as a “set of rules and regulations (written or unwritten) that does two things: it establishes and defines boundaries; and it tells you how to behave inside the boundaries to be successful.”(3) Barker reviews these concepts and their practical application in a 21-minute video.
Barker views paradigms as models used for problem-solving. For Barker, a “paradigm effect” is how you filter information so that it aligns with the paradigm you follow in dealing with explanations of any phenomena (e.g., Newtonian vs quantum physics) or following guidelines to accomplish a job-to-be-done (craft vs industrial woodworking). A paradigm shift is “changing from one set of rules to another.” I will apply concepts in Barker’s video to categorize and explain cell isolators’ different paradigms when purchasing and using collagenase products to isolate cells from tissue.
Three Collagenase Paradigms
Below, I identify and summarize three paradigms for using Clostridium histolyticum collagenase in cell isolation based on my knowledge of this field. The Common Beliefs are the rules that users follow to describe its boundaries. The Overlooked Issues are the “nagging facts” that emphasize the limitations of each paradigm.
1. “It’s All Good” Paradigm
Common Belief
- Traditional collagenase* is a purified enzyme with a tan to brown color
- Different collagenase types digest respective collagen types
- Always can find a good lot of traditional collagenase that works for my application
- Collagenase not critical because the number of cells recovered after isolation is more than what I need for my experiments
- Traditional collagenases has been used for years, why should I change?
Overlooked Issues
- Each lot has unique enzyme activities and “contaminating”** components
- Inconsistent isolation results lead many labs to “pre-qualify” lots before purchasing larger amounts of a “good” lot that lasts ≥ 1 year
- Assumes that critical enzyme activities are stable on storage
- Peers may be unable to replicate your results because different collagenase lots are used for cell isolation
*Traditional collagenase is the crude or partially enriched collagenase culture supernatants that have been sold for cell isolation since the 1960s. Typically it has a brown and tan color for crude and enriched collagenase, respectively.
** Contaminants refer to any other biochemical component in the collagenase product that does not have collagenase or protease activity.
The “it’s all good” paradigm is the most common based on the market share of traditional collagenase products used for enzyme-mediated cell isolation. I estimate an 80:20 split for the market shares of traditional:purified collagenase products. This assumption is based on my market analysis and also by analyzing the “hits” obtained when a proximity search is performed in Google Scholar for the past five years using the search term string “supplier name or Liberase AROUND(10) collagenase.” Traditional collagenase suppliers account for > 90% of the total hits versus those supplying purified collagenase enzymes.
Many users of traditional collagenase products ignore or fail to recognize that traditional collagenase products are crude biochemical products that contain a mixture of different enzyme activities. Several collagenase suppliers defined their collagenase products as “types.” Types can be defined by enzyme composition or their ability to isolate a specific cell population. As described below, optimal amounts of collagenase and neutral protease activities are required for tissue dissociation. The crude and enriched collagenase products contain between 3-8% and 15-25% collagenase per g dry weight, respectively. The brown color reflects the contaminating biopigment generated during the fermentation process. There is no correlation between the collagenase type and its ability to degrade a specific collagen type.
The enzyme activities in each product lot are unique since they reflect the strain of C. histolyticum used, the culture conditions of that specific fermentation, and the subsequent processing performed to manufacture the product. Furthermore, these enzyme activities can be affected at any step of the manufacturing process and during storage as a lyophilized product.(4, 5) Most users “pre-qualify” new lots of traditional collagenase before use. Once a “good” lot is found, that product is purchased to last a year or more before the pre-qualification process is repeated. VitaCyte’s white paper on “Lot Qualification” provides additional information on manufacturing and characterizing traditional collagenase products.
2. “Pure is Perfect” Paradigm
Common Belief
- Removal of contaminants by enzyme purification leads to a more consistent product
- Product consistency leads to avoidance or minimization to pre-qualify new lots of product
Overlooked Issues
- If care is not taken, collagenase’s collagen degradation activity (CDA) can be reduced during the purification process
- Assumes that CDA is stable on storage, although not measured
- Correlation of the effectiveness collagenase and protease in a mechanistic model for enzyme-mediated cell isolation not considered
The “Pure is perfect” paradigm arose after the Liberase™ Purified Enzyme Blend product line was developed at Boehringer Mannheim Biochemicals in 1995.(6) The first version of Liberase HI contained purified class I (C1) & class II (C2) collagenase and clostripain derived from C. histolyticum culture supernatants and Thermolysin from Bacillus thermoproteolyticus. Thermolysin was used instead of C. histolyticum neutral protease (CHNP) because of the inability to purify a stable, active protease.(7) Thermolysin and CHNP are members of the M4 protease enzyme family.(8)
Liberase HI addressed a significant hurdle for human islet isolators, offering enzyme mixtures specifically designed for human islet isolation. Before the introduction of this product, human islet isolators often spent more time trying to find a good lot of traditional collagenase than performing clinical research isolations to determine the effectiveness of islet transplantation in treating adult type 1 diabetic patients.
The first version of Liberase HI confirmed earlier observations that purified C. histolyticum collagenase and neutral protease gave the same islet yields as a good lot of a traditional collagenase product.(9, 10) Liberase HI was a unique product since about C1 & C2 collagenase comprised about 97% of the product.
C1 and C2 collagenases have complementary enzyme activities that result in the synergistic degradation of native collagen. C1 collagenase is a processive protease that “shaves” off tropocollagen molecules from collagen fibrils or fibers. In contrast, C2 collagenase is an endoprotease that creates collagen polypeptides. These differences in enzyme mechanism are shown by the specific activities of C1 and C2 when assayed for collagenase activity using collagen or peptide substrates. C1 has a 5 to 10 fold higher specific collagen degradation activity (CDA U/mg protein) in a kinetic fluorescent CDA assay than C2, whereas C2 has about a 50 fold higher specific activity using the Pz peptide (Wünsch U/mg protein) in the Wünsch assay than C1.(11-13)
The two endoproteases found in C. histolyticum culture supernatants, CHNP, and clostripain, also have complementary specificities. CHNP (and other M4 proteases) cuts on the amino-terminal side of sequences that contain Xaa + Yaa, in which Xaa is a hydrophobic residue, and Yaa is Leu, Phe, Ile, or Val. Clostripain is a trypsin-like serine protease that cuts on the carboxy-terminal side of arginine or lysine.(8)
Liberase HI was the “gold” standard for human islet isolation for at least ten years since it avoided the need for pre-qualifying collagenase before use.(14) The enzyme purity was > 95%, with minimal endotoxin contamination. Many, but not all, leading islet transplant centers adopted the product to use in their isolation procedure.
During 2002-2004, there was a change in the Liberase manufacturing process that led to lower human islet yields.(15) Lower yields resulted in “failed isolations” because the yield was below the amount needed for subsequent transplantation. Lower islet yields correlated with a change in the anion exchange chromatographic profiles of collagenase used to isolate these islets. There was no explanation for these results at the time of these reports, so Liberase HI remained the primary product used for human islet isolation.
In 2007, NIH forbade using Liberase products for isolating cells in any NIH-supported clinical trial. NIH’s concern was the potential risk of transmission of bovine spongiform encephalopathy since the Liberase culture media contained bovine brain-heart infusion.(14) Nordmark’s GMP Grade NB-1 Collagenase and NB Protease (CHNP), both derived from C. histolyticum, were used in place of Liberase HI for isolating human islets used in the Clinical Islet Transplantation Consortium (CITC) clinical trial.(7) Nordmark also used bovine animal products in their C. histolyticum culture media, but these products were pre-treated to minimize the risk of spongiform encephalopathy.
Most users successfully adopted the Nordmark enzymes, but the three most experienced islet transplantation centers could not achieve human islet yields similar to those obtained with Liberase HI.(7) VitaCyte analyzed Liberase HI, Nordmark NB-1 Collagenase, and their newly manufactured comparable, purified collagenase product, Collagenase HA, for:
- Collagenase Wünsch activity that measures C2 peptidase activity
- Collagen degradation activity using a newly developed kinetic fluorescent microplate CDA assay
- C1 degradation as detected using an analytical anion exchange chromatographic method
These results showed that decreased islet yields correlated with increased degradation of C1 collagenase(16). An earlier report showed C1 degradation was detected by the appearance of additional peaks after anion exchange chromatographic analysis, as shown in the Figure on the right.(17) Here, a purified preparation of C. histolyticum collagenase that contained partially degraded collagenase was passed over an anion exchange chromatography column.
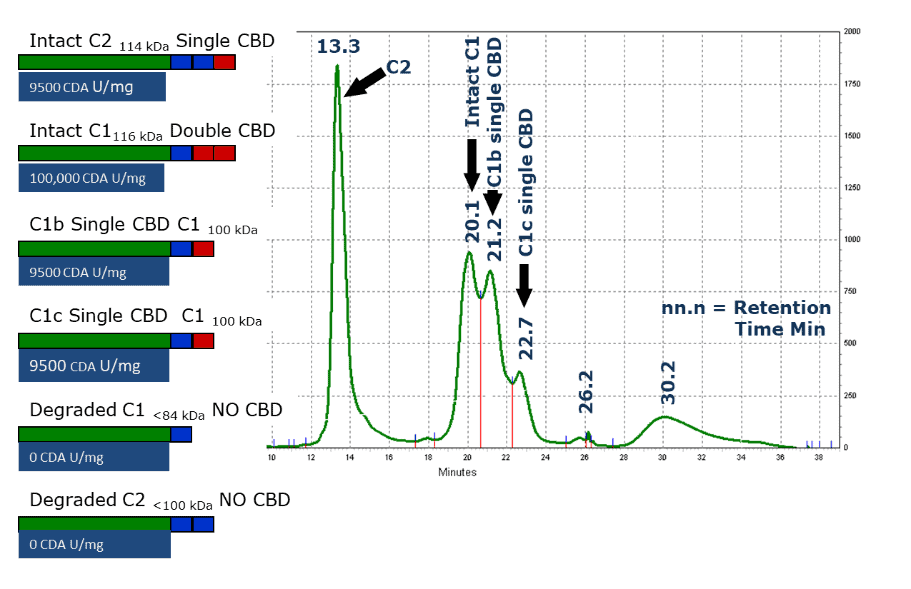
Peak column fractions were analyzed for CDA using the assays described above, and SDS-PAGE analysis to determine molecular mass. These results showed that intact C2 collagenase elutes first (13.3 min), followed by intact C1 (20.1 min). The third and fourth peaks elute at ≈ 1 and ≈ 2 minutes after the intact C1 peak, respectively, and are two different molecular forms of C1 collagenase (C1b and C1c) that lost ≈ the 16 kDa portion of the enzyme.
Sequencing of the C. histolyticum C1 and C2 genes and subsequent expression of these genes as recombinant proteins showed that C1 and C2 contained three functional domains: a catalytic domain that degraded/cut the collagen, a linking or PKD domain that has an amino acid sequence similar to a protein found in human polycystic kidney disease, and a collagen-binding domain (CBD). Intact C1 has a catalytic domain, a PKD domain, and two CBDs, whereas intact C2 has a catalytic domain, 2 PKD domains, and a single CBD. Further experiments showed that effective collagen degradation required that these enzymes contain a catalytic domain and at least one CBD.(11, 18-20)
The Figure above shows differences between specific CDA activity of intact C1, intact C2, and C1b and C1c molecular forms of collagenase. The forms with only one CBD have about 10% of the specific CDA of intact C1. These results were first interpreted by emphasizing the importance of the CBD. Still, more recent analysis shows that intact C1 and C1b/C1c forms have a 10 and 2.5 fold higher CDA-specific activity than C2. The latter data reflect the differences of C1 and C2 collagenase to degrade collagen.
The lower specific CDAs for the Nordmark products likely reflected the purification process used to manufacture their purified collagenase products. Nordmark could not detect degradation of C1 collagenase because they used a reverse chromatographic analysis to assess the purity of the collagenase product. This analysis does not detect the multiple C1 collagenase forms.(16)
The new insight linking collagenase structure-function to CDA led McCarthy et al. to propose a collagenase-protease-mediated cell isolation model.(7, 12) Here, the primary role of collagenase is to loosen the extracellular matrix (ECM) so that protease-sensitive sites on the ECMs “cell anchoring proteins” are cut, freeing the cells from the tissue. This model emphasizes the importance of having excess CDA to ensure that the normally “protease-resistant” ECM is compromised, enabling protease to cut the anchoring proteins, freeing cells from the ECM.
3. “Aligning Collagenase Function to Application” Paradigm
Common Belief
- CDA is the only assay that reflects collagenase function
- C1 and C2 synergistically degrade collagen: class I (C1) collagenase is a processive protease whereas class II (C2) is an endoprotease
- C1 can be cut by endoproteases (e.g., clostripain) likely reduces CDA activity and its ability to release cells from tissue
- If CDA is in excess, neutral protease activity dictates the speed of digestion, cell yield & viability, and damage to cell surface markers
Overlooked Issues
- Recognition of bias of most purified formulations based on reverse engineering “good” lots of collagenase
- Limited knowledge of the ideal collagenase-protease enzyme formulations, C1:C2 ratio influences total CDA used in the isolation procedure
- Optimal isolation procedure influenced by many parameters besides enzymes: delivery of enzyme to tissue, shaking/agitation, closed vs open digestion systems
The model for enzyme-mediated cell isolation proposed above was the basis for the new paradigm, “aligning collagenase function to application.” The model provides new insights identifying critical factors for successful collagenase-mediated cell isolation. These insights include:
- Excess collagenase will have a minimal adverse effect on cells, provided that
- intact C1 and C2 collagenase are the predominate forms in the mixture;
- minimal contamination of the purified collagenase by protease;
- clostripain activity is reduced to a very low level because this enzyme can degrade C1 collagenases during storage as a lyophilized product
- Collagenase does not damage cells since it has a restricted selectivity to degrade collagen, found only in the ECM
- Collagenase is critical for successful cell isolation since it degrades collagen, the protein that likely shields other ECM proteins from proteolysis
- Excess CDA must be used in cell isolation procedure to ensure “relaxation” of the ECM, leading to exposure of protease-sensitive sites on the ECM cell anchoring proteins
- Selection and dose of neutral protease used in an isolation procedure is critical since it affects the rate of cell release and recovery, cell viability, and damage to cell surface markers
- Use of two or more complementary proteases at an optimal dose in collagenase-protease enzyme mixtures will likely improve all the digestion parameters above
The practical application of the proposed model was published in an earlier blog post to explain the results from 94 human islet isolations using eight different enzyme mixtures performed at the University of Minnesota (UMN).(21) UMN performed these isolations to find an enzyme mixture superior to the Nordmark enzymes. This post shows how the new paradigm can be applied as a guidepost to interpret islet isolation results. The primary outcome of the UMN’s work was the development of a superior enzyme mixture for human islet isolation. The “new enzyme mixture” (NEM) contained VitaCyte’s Collagenase HA and Nordmark’s NB Protease.(21) The report found that the CHNP in NB Protease was superior to Thermolysin when used in collagenase-protease mixtures to isolate human islets. Collagenase-protease mixtures containing BP Protease gave similar or superior results to those islet isolations using the NEM.(7) These results emphasize the importance of focusing on the selection and dose of neutral protease in cell isolation to achieve the best results when using collagenase-protease mixtures to isolate cells.
In 2023, VitaCyte acknowledged the importance of rigorously characterizing collagenase used in cell isolation procedures by describing its collagenase products as “purified-defined.” VitaCyte uses three complementary assays to assess its C. histolyticum collagenase products: analytical exchange chromatographic analysis, Wünsch assay, and the kinetic fluorescent CDA assay. The first two assays are described in US Pharmacopeia monograph chapters <89.1> and <89.2> for C1 and C2, respectively, and characterization of the CDA assay is described above.
As shown above, analytical anion exchange analysis separates C1 from C2 collagenase and readily detects proteolysis of the carboxy-terminal C1 domain, generating truncated C1 (C1100 kDa) that is not as effective in degrading collagen as intact C1. Since the intact C1 contributes the bulk of the CDA in the kinetic fluorescent assay, this activity is a good measure of the total CDA in the sample. Conversely, assay of Wünsch activity is an excellent measure of the C2 collagenase. Results from the three analytical analyses provide a reproducible measure of the C1:C2 ratio, CDA, and the purity of the preparation.
References
1. Kuhn TS. Structure of Scientific Revolutions. Chicago: University of Chicago Press; 1962. 264 p.
2. Shorter Oxford Englsih Dictionary. Sixth ed. Oxford: Oxford University Press; 2007.
3. Barker JA. Paradigms, the Business of Discovering the Future. New York: Harper Collins; 1993. 240 p.
4. Cavanagh TJ, Lakey JR, Wright MJ, Fetterhoff T, Wile K. Crude collagenase loses islet-isolating efficacy regardless of storage conditions. Transplant Proc. 1997;29:1942-4.
5. Hefley T, Cushing J, Brand JS. Enzymatic isolation of cells from bone: cytotoxic enzymes of bacterial collagenase. Am J Physiol. 1981;240:C234-8.
6. Fetterhoff TJ, Cavanagh TJ, Wile KJ, Wright MJ, Dwulet FE, Gill J, et al. Human pancreatic dissociation using a purified enzyme blend. Transplant Proc. 1995;27:3282-3.
7. McCarthy RC, Green ML, Dwulet FE. Evolution of enzyme requirements for human islet isolation. OBM Transplantation. 2018; 2:[1-30 pp.]. Available from: http://www.lidsen.com/journals/transplantation/transplantation-02-04-024.
8. Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic acids research. 2010 2010;38: D227-D33. Available at https://www.ebi.ac.uk/merops/.
9. Hefley TJ, Stern PH, Brand JS. Enzymatic isolation of cells from neonatal calvaria using two purified enzymes from Clostridium histolyticum. Exp Cell Res. 1983;149:227-36.
10. Hatton MW, Berry LR, Krestynski F, Sweeney GD, Regoeczi E. The role of proteolytic enzymes derived from crude bacterial collagenase in the liberation of hepatocytes from rat liver. Identification of two cell-liberating mechanisms. Eur J Biochem. 1983;137:311-8.
11. McCarthy RC, Sakon JS. Webinar: Collagen degradation by Clostridal histolyticum collagenases. 2021 [Available from: Collagen Degradation by Clostridial histolyticum Collagenases – VitaCyte (cloudwaysapps.com).
12. McCarthy RC BA, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: Factors to consider in setting enzyme acceptance criteria. Transplant. 2011;91:137-45.
13. Eckhard U, Schönauer E, Nüss D, Brandstetter H. Structure of collagenase G reveals a chew-and-digest mechanism of bacterial collagenolysis. Nature Struct Mol Bio. 2011;18:1109-14.
14. Wang Y, Paushter D, Wang S, Barbaro B, Harvat T, Danielson KK, et al. Highly purified versus filtered crude collagenase: comparable human islet isolation outcomes. Cell Transplant. 2011;20:1817-25.
15. Barnett MJ, Zhai X, LeGatt DF, Cheng SB, Shapiro AMJ, Lakey JRT. Quantitative assessment of collagenase blends for human islet isolation. Transplant. 2005;80:723-8.
16. Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplant. 2010;89:954-61.
17. McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, Dwulet FE. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplant Proc. 2008;40:339-42.
18. Bauer R, Janowska K, Taylor K, Jordan B, Gann S, Janowski T, et al. Structures of three polycystic kidney disease-like domains from Clostridium histolyticum collagenases ColG and ColH. Acta Crystallographica Sect D, Biological Crystallography. 2015;71(Pt 3):565-77.
19. Matsushita O, Jung CM, Katayama S, Minami J, Takahashi Y, Okabe A. Gene duplication and multiplicity of collagenases in Clostridium histolyticum. J Bacteriol. 1999;181:923-33.
20. Eckhard U, Brandstetter H. Polycystic kidney disease-like domains of clostridial collagenases and their role in collagen recruitment. Biological Chemistry. 2011;392:1039-45.
21. Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplant. 2012;93:693-702.
