
Occam’s razor is a useful mental model to develop hypotheses to untangle complex problems. It can be summarized as follows:
“Other things being equal, simpler explanations are generally better than more complex ones.”
In 2011, McCarthy et al. proposed a hypothetical model of tissue dissociation that focused on how the synergistic interaction of collagenase and protease enzyme activities led to the release of cells from tissue.(2) An article published recently summarized key postulates of this model. This model is based on the following facts:
- The extracellular matrix (ECM) is a jungle of proteins where collagen is the predominate protein in the ECM(3)
- The ECM is resistant to exogenous protease since there is minimal release of cells from tissue using protease alone(4, 5)
- Collagen’s triple helical structure is resistant to proteolytic degradation; the only enzymes that can effectively degrade the helical structure are defined as functional collagenases(6)
- Clostridium histolyticum has two classes of collagenase, class I (C1) and class II (C2), that have complementary selectivity to work synergistically to degrade native collagen(7)
- Only those forms of C1 or C2 with a catalytic domain and at least one collagen binding domain can effectively degrade collagen(7)
- The assays commonly used to measure collagenase activity (e.g., Wunsch, FALGPA, gelatinase) measure both functional and non-functional collagenase (i.e., non-functional collagenase has no collagen binding domains)(2)
- Collagenase has restricted selectivity: it degrades only native collagen or denatured collagen (i.e., gelatin); gelatinase activity only requires a functional catalytic domain(2)
- Collagenase and neutral protease activity is required to release cells from tissue(4,5)
These facts are included in the model with the following unproven but reasonable assumptions:
- Collagen provides a protease-resistant superstructure that protects the ECM proteins from proteolytic degradation
- Disruption of the collagen structure in the ECM leads to the loosening of the matrix, exposing protease-sensitive sites on the ECM proteins, enabling proteases to degrade these proteins
- Once a sufficient number of “cell anchoring” ECM proteins are degraded, cells are released from tissue
The model can troubleshoot problems, interpret data, or develop new enzyme formulations for cell isolation methods. The model requires that collagenase degradation activity is in excess and that an appropriate dose of one or more proteases is used in cell isolation. The addition of excess purified collagenase is unlikely to harm the isolated cells since its selectivity is restricted to collagen and gelatin.
To illustrate an application of this model, data reported by the University of Minnesota (UMN) to find a collagenase-protease enzyme mixture to replace Liberase HI for human islet isolation will be reviewed.(8) This search was driven by the Clinical Islet Transplantation Consortium, which forbade using Liberase HI to isolate and recover islets for subsequent transplantation in their upcoming clinical trial because of the potential transmission of spongiform encephalitis.
The UMN report assessed the effectiveness of eight different enzyme mixtures in recovering islets from human donor pancreata. These mixtures used the three functional collagenase isoforms: intact C1 (C1116 kDa), truncated C1 (C1100 kDa), and intact C2 (C2114 kDa), and either thermolysin or C. histolyticum neutral protease (ChNP). The table below summarizes the ability of these three isoforms to degrade native collagen in a kinetic, fluorescent microtiter plate collagen degrading activity (CDA) assay.
| Intact C1:C1116 kDa | Truncated C1:C1100 kDa | Intact C2:C2114 kDa | |
| CDA U/mg protein (n) | 134,739 (20) | 53,185 (12) | 13,320 (15) |
| Normalized as % intact C1 | 100 | 39.5 | 9.9 |
Truncated C1 (C1100 kDa) lost the carboxy-terminal collagen binding domain by proteolysis and has about 35% of the CDA of C1116 kDa isoform with two collagen binding domains. Those collagenase isoforms with a single collagenase binding domain (C1100 kDa, C2114 kDa) are less effective for degrading collagen than the C1116 kDa isoform with two collagen binding domains.(2)
Below is a graph and table summarizing the results from 94 human islet isolations performed using eight different enzyme mixtures noted above. The islet isolation groups are split based on the predominance of C1116 kDa or C1100 kDa used in the enzyme mixture. The collagenase dose was set at > 1800 Wunsch Units per isolation in all these isolations. It is assumed that there is sufficient C2114 kDa in the enzyme mixtures since Wunsch activity measures functional or non–functional C2 collagenase activity. Moreover, C2 is not as quickly degraded by protease as C1.
The Serva-Nordmark (S-N) purified collagenase manufactured at that time contained predominantly C1100 kDA.(9) The islet equivalent number counts (IEQs) for Groups 1 to 3 were low, ranging from 1603 to 2202 IEQ/g tissue for enzyme mixtures containing ChNP or thermolysin. The results from Group 4 show that if the isolations were performed with collagenase lots with higher trypsin-like activity, islet yield increased by 46% compared to those obtained in Group 1.
Groups 5-8 used a 60:40 C1:C2 purified collagenase enzyme mixtures where the C1 contained predominantly C1116 kDa mixed with thermolysin or ChNP. Groups 5 & 7 had islet yields comparable to Group 1. Group 7 had a higher CDA-specific activity, reflecting an increase in C1116 kDa than found in enzyme mixtures used in Group 6. An earlier report showed that the enzyme formulation used in Group 6 gave significantly better islet isolation results when compared to those using the S-N collagenase-protease mixtures, emphasizing the importance of using C1116 kDa for isolating islets using the Ricordi method.(9) The Group 6 results were comparable to the Group 4 results indicating that the addition of increased clostripain activity may improve islet yields, as shown by others. However, the best yields were obtained when C1116 kDa– C2114 kDa mixtures were used with S-N’s NB Protease. The protease activity was ≥ 1.5 DMC U/g tissue. The results from Group 8 were significantly better than those for Groups 1-6 for digestion time, digest IEQ, digested IEQ per g pancreas, post-purification total IEQ, and post-purification IEQ per g pancreas.
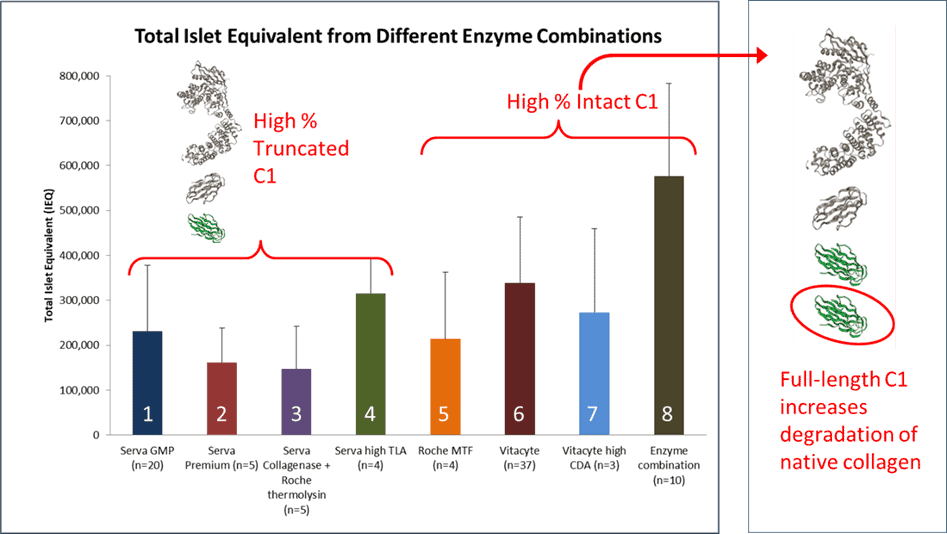
| Group #: Supplier(s) | Protease | Number Isolations | Total IEQ Post-Purification |
IEQ/g Tissue Post-Purification |
| 1: Serva-Nordmark (S-N) GMP Grade | ChNP | 24 | 2202 ± 1403 | 231,952 ± 145,844 |
| 2: S-N Premium Grade | ChNP | 5 | 1635 ± 726 | 161,641 ± 145,844 |
| 3: S-N + Roche Thermolysin | Thermolysin | 5 | 1603 ± 965 | 146,605 ± 95,676 |
| 4: S-N high TLA | ChNP | 4 | 3223 ± 855 | 315,599 ± 77,064 |
| 5: Roche MTF | Thermolysin | 4 | 2217 ± 1405 | 214,276 ± 148,227 |
| 6: VitaCyte | Thermolysin | 37 | 3467 ± 1698 | 338,623 ± 147,061 |
| 7: VitaCyte | Thermolysin | 3 | 2147 ± 1299 | 272,766 ± 187,426 |
| 8: VitaCyte/S/N = New Enzyme Mixture (NEM)* | ChNP | 12 | 5329 ± 2519 | 549,938 ± 246,131 |
Applying the enzymatic tissue dissociation model to interpret these results emphasizes the importance of using C1-C2 collagenase-protease enzyme mixtures with excess collagen degradation activity and an appropriate neutral protease at an optimal dose.(8) The results above show that Thermolysin-containing collagenase-protease enzyme mixtures were less effective than ChNP for human islet isolation. A separate set of islet isolations were performed to confirm this observation. Here, enzyme mixtures containing VitaCyte’s collagenase combined with thermolysin or NB Protease were compared within the same organ by performing separate digestions on the head-body or body-tail of the pancreas.(8) To minimize the anatomical difference in islet number between the two portions, the digestions were alternated sequentially between the two tissue portions. These results showed that enzyme mixtures using NB Protease gave significantly higher islet counts with larger islets when compared to islets isolated with thermolysin.
The NEM has been used for human islet isolations at UMN since 2009. Further reports have shown that BP Protease (a purified Dispase equivalent enzyme) is as effective or superior to ChNP for human islet isolation.(10) This product has a higher purity than the ChNP NB Protease product.(11)
In closing, the model of tissue dissociation would also predict that the incorporation of two or more proteases with complementary selectivity is likely to improve islet recovery. The inclusion of two complementary proteases with different selectivity will accelerate the degradation of the tissue, shortening the digestion time. The inclusion of clostripain with BP Protease and collagenase for porcine islet isolation decreased the digestion time and percentage of undigested tissue when compared to the same enzyme mixtures without clostripain (M Green unpublished observations).
The results from human islet isolations using collagenase and protease supplemented with clostripain have been inconsistent.(12, 13) These inconsistent observations may reflect poor characterization of collagenase used in these studies or the likely increase in endogenous pancreatic protease activity caused by clostripain in the collagenase or neutral protease. Clostripain’s trypsin-like enzyme activity will convert pancreatic zymogens to active proteases.(2)
In conclusion, developing a robust islet isolation method requires the end users to be vigilant, using properly characterized collagenase or protease enzymes. In 2011, McCarthy et al. (2) proposed that collagenase should be characterized using 3 assays:
- Analytical anion exchange chromotography to assess degradation of the C1 or C2
- Wunsch activity to assess the amount of C2
- CDA using a kinetic, fluorescent microtiter plate assay
In addition, unactivated (i.e., trypsin-like activity) and activated clostripain activity should be added to these analyses because variable amounts of this activity in different product lots can increase cell yields.
The first two methods are described in US Pharmacopeia monograph Chapters <89.1> and <89.2> for C1 and C2 collagenase, respectively. The Mono-Q analytical anion exchange column is no longer manufactured by Cytiva. VitaCyte is evaluating Cytiva’s replacement product and will update our progress on this evaluation in a later report. VitaCyte is also working with others to transfer its kinetic fluorescent microtiter plate assay so it can be used for the evaluation of collagen degradation activity. If interested in working with VitaCyte staff on this project, contact Bob McCarthy.
References
- Occam’s razor Wikipedia: Wikimedia Foundation Inc; [Available from: https://en.wikipedia.org/wiki/Occam%27s_razor.
- McCarthy RC BA, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: Factors to consider in setting enzyme acceptance criteria. Transplantation. 2011;91:137-45.
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. Journal of Cell Science. 2010;123(Pt 24):4195-200.
- Hatton MW, Berry LR, Krestynski F, Sweeney GD, Regoeczi E. The role of proteolytic enzymes derived from crude bacterial collagenase in the liberation of hepatocytes from rat liver. Identification of two cell-liberating mechanisms. Eur J Biochem. 1983;137:311-8.
- Hefley TJ, Stern PH, Brand JS. Enzymatic isolation of cells from neonatal calvaria using two purified enzymes from Clostridium histolyticum. Experimental Cell Research. 1983;149:227-36.
- McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, et al. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplantation Proceedings. 2008;40:339-42.
- Matsushita O, Okabe A. Clostridial hydrolytic enzymes degrading extracellular components. Toxicon. 2001;39:1769-80.
- Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93:693-702.
- Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010;89:954-61.
- McCarthy RC, Green ML, Dwulet FE. Evolution of enzyme requirements for human islet isolation. OBM Transplantation. 2018; 2:[1-30 pp.]. Available from: http://www.lidsen.com/journals/transplantation/transplantation-02-04-024.
- AG Breite FD, RC McCarthy. Purification and characterization of Clostridium histolyticum neutral protease used for human islet isolation. 2010; XXIII International Congress of the Transplantation Society; Vancouver BC.
- Brandhorst H, Friberg A, Andersson HH, Felldin M, Foss A, Salmela K, et al. – The importance of tryptic-like activity in purified enzyme blends for efficient islet isolation. Transplantation. 2009;87:370-5.
- Stahle M, Foss A, Gustafsson B, Lempinen M, Lundgren T, Rafael E, et al. Clostripain, the Missing Link in the Enzyme Blend for Efficient Human Islet Isolation. Transplantation Direct. 2015;1:e19.
