The development of the Liberase™ HI Purified Enzyme Blend product by an R&D team that included VitaCyte’s (VC’s) co-founders led to the company’s keen interest in optimizing the enzyme composition and dose used for human islet isolation. Several years ago, VC published a technical poster summarizing laboratory results that compared the performance of commercial collagenase-protease enzyme mixtures used for human islet isolation. This table included data not found in the original publications because McCarthy contacted the authors to close the information gaps in these reports.
Entries 8 & 10 from the above technical poster summarized studies performed by AN Balamurugan and colleagues where they evaluated the effectiveness of recombinant class I (rC1) and class II (rC2) C. histolyticum collagenase combined with BP Protease to isolate human islets. Entry 8 summarized results from the University of Minnesota, where VC used funds from a Phase II SBIR award to perform a design of experiment (DOE) study to determine the impact of dose of rC1 and rC2 collagenase on human islet recovery.(1) This study showed that enzyme mixtures of rC1 at 100,000 or 200,000 CDA U mixed with either 12 or 20 Wunsch U of rC2 and BP Protease and a fixed dose of 23,400 neutral protease U (NP U) per g tissue, showed no differences in islet recovery. This was a surprising finding because we believed the lower doses of rC1 or rC2 would lead to suboptimal islet recovery.
The second report (entry 10) summarized results that used the lowest dose of rec collagenase in the DOE study above (100,000 CDA U rC1, 12 WU rC2, and 23,400 NP U of BP Protease, rC1:rC2 = 30:70).(2) These results indicated that islets isolated with rec collagenase had better functional activity than islets isolated with natural purified collagenase (Collagenase HA) when both enzyme mixtures used the same dose of BP Protease activity. Moreover, reducing the Collagenase HA dose from 20 to 12 WU of C2 per g tissue (CDA from 240,480 to 144,000 CDA U per g tissue) mixed with the same BP Protease activity resulted in ≈ 50% decrease in islet yield.
At the last IPITA meeting, O’Gorman and colleagues at the University of Alberta in Edmonton reported results from a large series of islet isolations comparing VitaCyte’s recombinant collagenase (rCollagenase HI) – BP Protease enzyme mixture to natural purified collagenase and thermolysin mixtures manufactured by either VitaCyte or Roche.(3) The modified table from this abstract summarizes these results:
| Parameter | Collagenase HA & Thermolysin (n=109) | Collagenase MTF & Thermolysin (n= 229) | rCollagenase HI & BP Protease (n=49) | p value |
| Collagenase Dose WU/g | 28.8 ± 0.7 | 31.5 ± 0.5 | 16.8 ± 0.1 | < 0.001 |
| Neutral Protease U/g | 15,716 ± 1,068 | 19,740 ± 1,040* | 21,044 ± 1,393 | ND |
| Pre-Purify IE (x103) | 554 ± 24 | 573 ± 17 | 580 ± 44 | 0.775 |
| Post-Purify IE (x103) | 400 ± 19 | 409 ± 15 | 457 ± 38 | 0.318 |
| Post-Purify IPN (x103) | 329 ± 13 | 335 ± 11 | 418 ± 33 | 0.005 |
| Post-Culture IE (x103) | 487 ± 23 | 500 ± 15 | 594 ± 42 | 0.020 |
| Post Culture IPN (x103) | 397 ± 15 | 414 ± 13 | 515 ± 34 | 0.001 |
| Culture Recovery % | 89.1 ± 0.9 | 88.9 ± 1.2 | 94.1 ± 2.2 | 0.112 |
| Viability % | 86.1 ± 0.9 | 87.9 ± 0.6 | 88.0 ± 1.4 | 0.242 |
| Transplant Dose IEQ/kg bw | 6,659 ± 314 | 6902 ± 204 | 8,021 ± 513 | 0.037 |
*Roche NP U converted to VitaCyte (VC) NP U using a conversion factor of 19.26 derived from the following calculation: specific activity of Roche thermolysin found in C of A led to mg thermolysin used, which was then multiplied by 260,000 that is, the specific activity of purified Thermolysin in VCs neutral protease assay
These data show that equivalent results were obtained using VC’s Collagenase HA and Thermolysin or Roche’s MTF kit. These two products are comparable since the C. histolyticum subclones used to generate the raw material are from the same parent strain and both a formulated with a 60:40 C1:C2 ratio. The major differences are the selection of the subclone, the choice of gelatin used to stimulate collagenase production, and the method of collagenase purification. Both products use Thermolysin supplied by the same vendor. The primary difference is the assignment of mass based on the use of either 1.76 or 1.1 A280 U per mg of protein.
A comparison of the three enzyme mixtures showed that all three groups used similar neutral protease activities per g tissue. The notable difference in these enzyme mixtures was the ability of rCollagenase HI to isolate islets effectively using ≈ 50% less collagenase.
Comparison of the islet parameters from each enzyme mixture showed that islets isolated with rCollagenase HI gave higher post-purify IPN counts, post-culture IPN, or IE counts, resulting in a significantly higher mass of islets transplanted into the recipients.
The other interesting finding from this report was the significantly higher insulin response to glucose challenge by islets isolated with rCollagenase HI-BP Protease compared to those isolated with natural collagenase and Thermolysin. The bar graph below shows glucose-stimulated insulin release analysis results, expressed per IPN or IE.
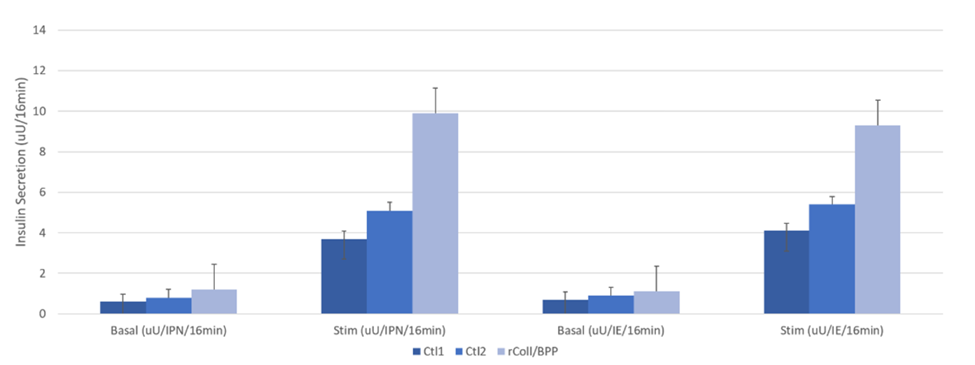
A comparison of the clinical islet transplant outcomes one month after transplant showed no differences in the Beta 2 score or post-transplant insulin production between the three groups on islet products.
If you know of any other reports that should be added to the human islet yield table, please provide the citation to Bob McCarthy (rcmccarthy@vitacyte.com). This report will be included in an updated human islet yield table after receiving several additional entries.
- Balamurugan AN, Green ML, Breite AG, Loganathan G, Wilhelm JJ, Tweed B, et al. Identifying Effective Enzyme Activity Targets for Recombinant Class I and Class II Collagenase for Successful Human Islet Isolation. Transplantation Direct. 2016;2(1):e54.
- Loganathan G, Subhashree V, Breite AG, Tucker WW, Narayanan S, Dhanasekaran M, et al. Beneficial effect of recombinant rC1rC2 collagenases on human islet function: Efficacy of low-dose enzymes on pancreas digestion and yield. American Journal Transplantation. 2018;18:478-85.
- O’Gorman D, Kin T, Rosichuk S, Richer B, Zhai W, Moriarity J, et al., 2021. Transplantation . Evaluation of a low dose recombinant collagenase and BP Protease for clinical islet transplantation. 2021 International Pancreas and Islet Transplantation Association; https://journals.lww.com/transplantjournal/Fulltext/2021/12001/306_4__Evaluation_of_a_Low_Dose_Recombinant.30.aspx
