The transition of enzyme suppliers in 2007 from Liberase™ HI to Nordmark/Serva’s NB-1 collagenase & NB protease for isolation of human islets used in Collaborative Islet Transplantation consortium clinical trial was problematic for some expert islet labs but not for others. During this period, the number of allo-islet transplantations performed in North America was at its lowest level over the period from 2001 to 2013.(1) This transition led many labs to modify their procedures or find other sources of enzyme. How were Nordmark’s enzymes different than Liberase™ HI? A report published in 2010 showed this difference might, in part, be due to the molecular form of class I (C1) collagenase found in the enzyme mixture. Nordmark’s enzyme mixtures, containing truncated C1, gave significantly lower islet yields and longer digestion times than VitaCyte enzyme mixtures that contained primarily intact C1.(2) A recent report by VitaCyte scientists confirms these observations by making direct comparisons of the ability of two enzyme mixtures that differ only in the molecular form of C1 to recover islets from an adult split porcine pancreas.(3) Before reviewing these results, the overview presented below reviews basic knowledge on correlation of collagenase structure-function to collagen degradation activity.
C. histolyticum Collagenase Structural Correlation with Specific CDA
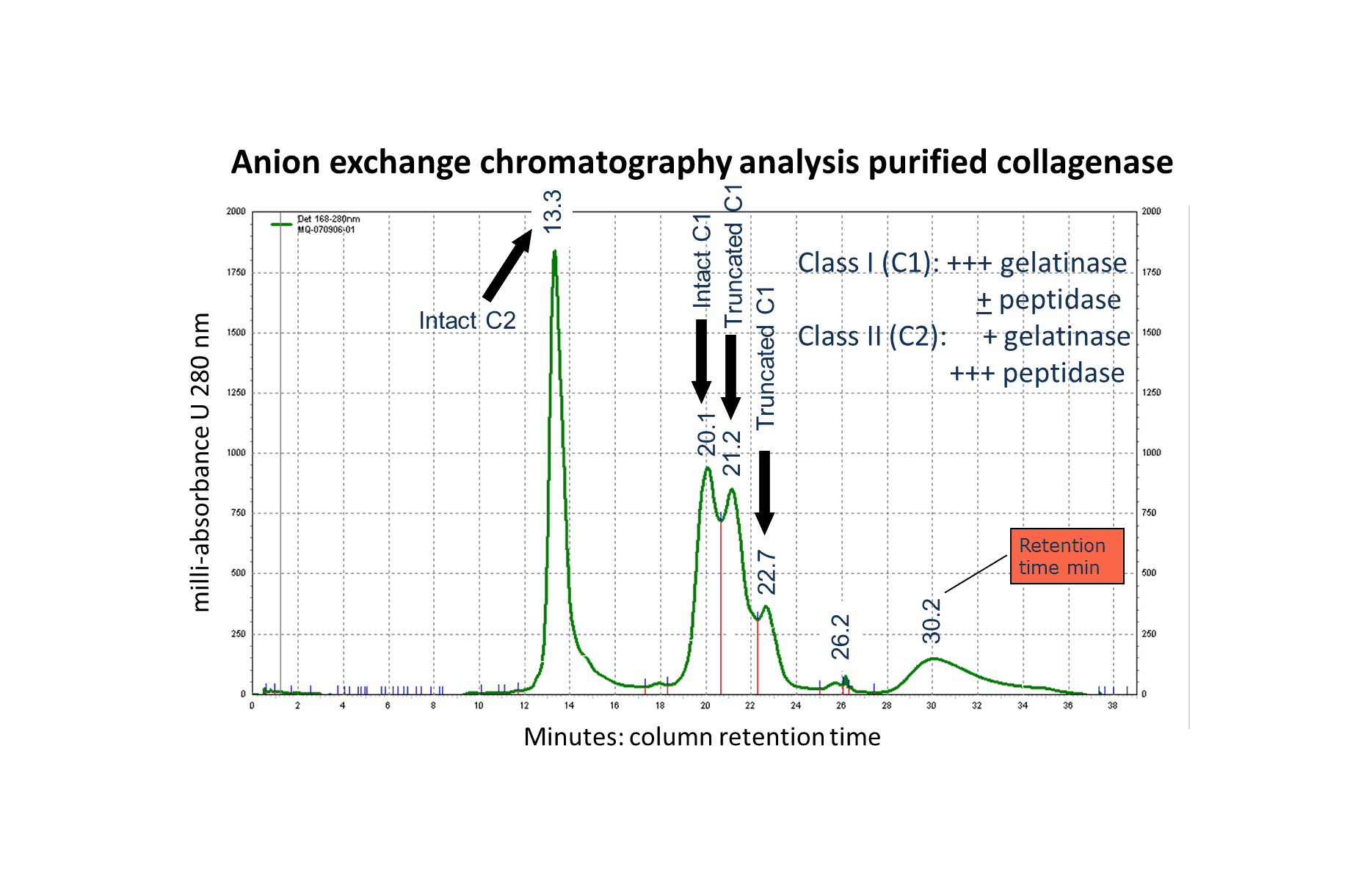
Enzyme mixtures containing C. histolyticum class I (C1) & class 2 (C2) collagenase and neutral protease are required to release islets from human pancreas. C1, C2, and protease work cooperatively to degrade the extracellular matrices

containing collagen fibrils or fibers.(4) Prior studies using natural, purified C1 or C2 correlated collagen degradation activity (CDA) assay results with the C. histolyticum protein structure.(5) Intact C1 with two collagen binding domains (CBDs) had about a 7 fold higher specific CDA (CDA U/mg protein) than those forms that contained a single CBD (see Figure to the right). The function of the catalytic and CBDs are self-explanatory, the function of the linking domain is unknown. Only collagenase containing a catalytic and at least one CBD can degrade native collagen.(4)
Removal of the carboxyl-terminal collagen binding domain by proteolysis during the manufacturing process likely created truncated C1. The term truncated is used in place of degraded since the loss of one of the two collagen binding domains on C1 does not affect its ability to degrade native collagen. Loss of both collagen binding domains on C1 or the sole collagen binding domain on C2 leads to molecular forms that cannot degrade native collagen.
Mass of C1 Required for Successful Porcine Islet Isolation Dependent on Molecular Form of C1 Enzyme
A recent report from VitaCyte’s scientists showed the truncated form of recombinant C1 collagenase was less efficient than recombinant intact C1 in releasing islets from tissue.(3) Two enzyme mixtures with nearly identical enzyme target activities were prepared. These targeted activities were CDA for C1, Wunsch activity for C2, and neutral protease activity for BP Protease. The only difference between these enzyme mixtures was that one used intact C1 whereas the other used truncated C1. Each mixture was alternated between the head and body-tail of a split adult porcine pancreas to minimize anatomical bias and allowed comparisons within the same organ.
The results from 5 direct comparisons showed that each enzyme mixture gave comparable islet yields (999 ± 242 and 1044 ± 331 for mixtures containing truncated C1 and intact C1, respectively). However, about 13 fold higher amounts of recombinant truncated C1/g tissue were required to achieve the same islet yields as found in enzyme mixtures containing intact rec C1. The switch time, percent undigested tissue, islet equivalents (IEQ) per g tissue and packed tissue volume per g tissue were not significantly different.
Relevance of this Observation to Human Islet Isolation
This report is the first direct comparison of differences in the effectiveness of the molecular form of C1 to recover porcine islets.(3) This conclusion is also applicable to isolation of human islets.
Analysis of 90 human islet isolations using 8 different enzyme mixtures showed that a “new enzyme mixture” composed of purified, natural, intact C1 and C2 (CIzyme Collagenase HA), mixed with an enriched C. histolyticum neutral protease (NB Protease) gave significantly shorter digestion times and higher post purification yields when compared to those isolations using Premium or GMP grade NB-1 Collagenase and NB Protease from Nordmark.(6) The earlier report cited above showed that these enzymes contained primarily truncated C1.(2) [Nordmark has since improved their NB-1 Collagenase product by increasing the amount of intact C1 as a percentage of total C1 (VitaCyte, unpublished data)].
As discussed in earlier reports(3, 4) and blog posts(7), collagenase products used for human islet isolation should contain primarily intact C1 and C2 because these molecular forms have the highest specific CDA (CDA U/mg protein). If this condition is not met, variability in CDA may lead to suboptimal collagen degradation, leading to a higher percentage of undigested tissue and embedded islets, that in turn results in lower islet yields. Basing collagenase dose on Wünsch activity has been shown not to correlate with islet yield.(8)
Excess CDA must be used in islet isolation to ensure maximal degradation of native collagen since it is unlikely to have an adverse effect on islet viability, yield, or function for two reasons. Collagenase has a narrow specificity, degrading only native and denatured (i.e., gelatin) collagen and the extracellular matrix is a complex jungle of native collagen and other proteins that hold cells to tissue. Neutral protease activity is needed to break down this complex structure by accelerating the degradation of denatured collagen that in turn loosens the matrix leading to proteolytic degradation of proteins that hold cells to the tissue. Selection of the type and dose of protease should be the focal point for further study assuming that an acceptable collagenase product is used for the islet isolation procedure.
In the next blog post, “Collagenase is a critical process parameter for human islet isolation,” I will summarize results from a recent paper from the University of Louisville group that provides a new perspective on how collagenase dose may affect islet function.
References
- Collaborative Islet Transplant Registry 2016; 9th Annual Report. http://www.citregistry.org.
- Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010;89(8):954-61.
- Green ML, Breite AG, Beechler CA, Dwulet FE, McCarthy RC. Effectiveness of different molecular forms of C. histolyticum class I collagenase to recover islets. Islets. 2017:e1365996.
- McCarthy RC BA, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: Factors to consider in setting enzyme acceptance criteria. Transplantation. 2011;91:137-45.
- McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, et al. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplantation proceedings. 2008;40(2):339-42.
- Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93(7):693-702.
- McCarthy RC. Does collagenase truly deserve top billing as the key enzyme for isolating primary cells? 2017 [Available from: https://www.vitacyte.com/news/does-collagenase-truly-deserve-top-billing-as-the-key-enzyme-for-isolating-primary-cells/.
- Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. American Journal of Transplantation. 2007;7(5):1233-41.
