Quality AssuranceQuality Assurance as the Foundation of Value
At VitaCyte, Quality Assurance means more to us than simply meeting a set of standards or requirements. For us, Quality Assurance is the foundation for our unique value not only to our customers — as an enzyme supplier and trusted collaborators — but also to the body of scientific work our customers seek to expand and refine. We organize that value around three pillars of excellence.
1
Superior Enzyme Performance
We strive to produce enzymes that perform consistently, with superior cell yield, viability, and functionality. We provide certificates of analysis with all our products so you know what you’re getting.
2
Manufacturing Excellence
Because we leverage the Quality by Design Philosophy, we make manufacturing excellence a top priority. We not only employ a detailed, precise, and methodical approach to our manufacturing process, but we also pay careful attention to our packaging for ease of use.
3
Commitment to Continuous Improvement
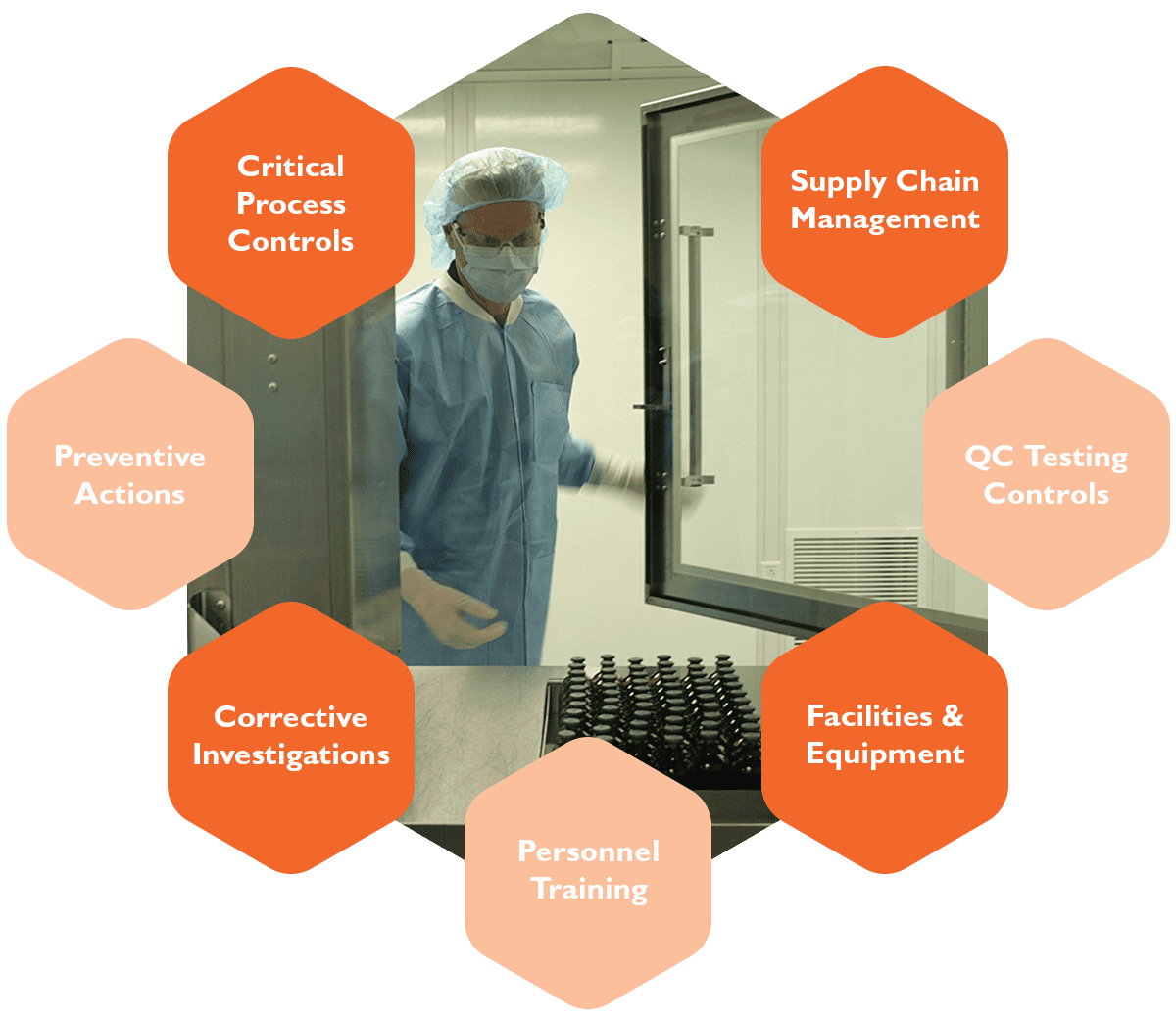
Our commitment to continuous improvement anchors our robust and controlled manufacturing process. We apply critical process controls, corrective and preventive investigation, and QC testing controls — as well as rigorous supply chain management and personnel training.
VitaCyte’s Quality Management SystemRobust Controlled Manufacturing Process
Our rigorous Quality Management System was developed and implemented under the direction of experienced API manufacturing QA professionals. From the enzymes used for research and clinical trials to those employed for clinical application, our robust manufacturing process is tightly controlled and focused on producing quality product. Specifically, our manufacturing is performed in accordance with with principles for clinical trial material outlined in the International Conference on Harmonisation (ICH) document ICH Q7a “Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients.” In addition, our document control is aligned with FDA guidance for Phase I material as described in 21 CFR Parts 210 and 211.
Our Approach to Quality Assurance Gets Results
We consider a robust, controlled manufacturing process as part of our approach to customer care. Our methodical approach to QA and manufacturing, coupled with our scientific expertise, make us the dependable, qualified supplier our customers have come to rely on.

