Andrew G. Breite
Two published Standards on ancillary materials (AMs) are the US Pharmacopeia (USP) <1043> and ISO 20399 International Standard. The ISO standard defines AMs as “materials that come in contact with the cellular therapeutic product during cell processing but are not intended to be part of the final product formulation.” AMs are not regulated like cell and gene therapies (CGT), where applications to regulatory authorities contain rigorous descriptions for the manufacture, validation, and characterization of the cell therapeutic product.
The key sections of each Standard are compared in the table below. The USP chapter focuses primarily on CGT manufacturers’ risk-based approach to qualifying AM suppliers and products. The ISO Standard goes further by outlining requirements the AM manufacturer should conform to for qualification as a supplier. Both Standards emphasize the importance of a CGT manufacturer having a robust supplier qualification program that establishes acceptance criteria for the AM supplier. As an AM supplier, VitaCyte has developed a robust quality system designed to help CGT manufacturers efficiently qualify the company as a reliable supplier.
Both Standards emphasize the understanding, documenting, and managing known aspects of the CGT manufacturing process that impact quality and consistency. Certain complex AMs, such as media and enzymes, can have a profound impact on the CGT product, which makes users responsible for understanding how these products relate to the CGT’s critical quality attributes (CQA). The AM supplier is responsible for controlling, documenting, and communicating known aspects of the AM manufacturing process that impact the quality and consistency of the product. VitaCyte has designed manufacturing processes and product testing that align with the criteria that impact AM performance for the intended application. For example, the use of collagen degrading activity assay is relevant to users as this assessment directly measures the ability of collagenase products to degrade native collagen in the extracellular matrix.
The foundation for qualifying the AM products is to collect supporting documentation commensurate with the risk of the product. In addition, the risk assessment must assess the variability of the AMs on the final product. The USP offers a tier-based system for AM products and offers recommendations for the level of product documentation required to qualify the product in the application. While the burden for this assessment lies with the user, using defined and consistent enzyme reagents will help facilitate this process. Consistent with these Standards, VitaCyte uses manufacturing processes and product designs intended to minimize lot variability.
Both Standards suggest that supplier-generated documents provide adequate information to mitigate the user’s risks. This is best accomplished by developing a close partnership between CGT manufacturers and their AM suppliers. AM suppliers should be transparent about the features of their products that help users mitigate risk to the CGT manufacturing process. However, this two-way relationship works best when users share their acceptance criteria and risk management expectations. VitaCyte works proactively to cultivate relationships with users to meet its responsibility as a trusted AM supplier, consistent with these Standards.
As an introduction, a high-level comparison between the two Standards is shown in the table below.
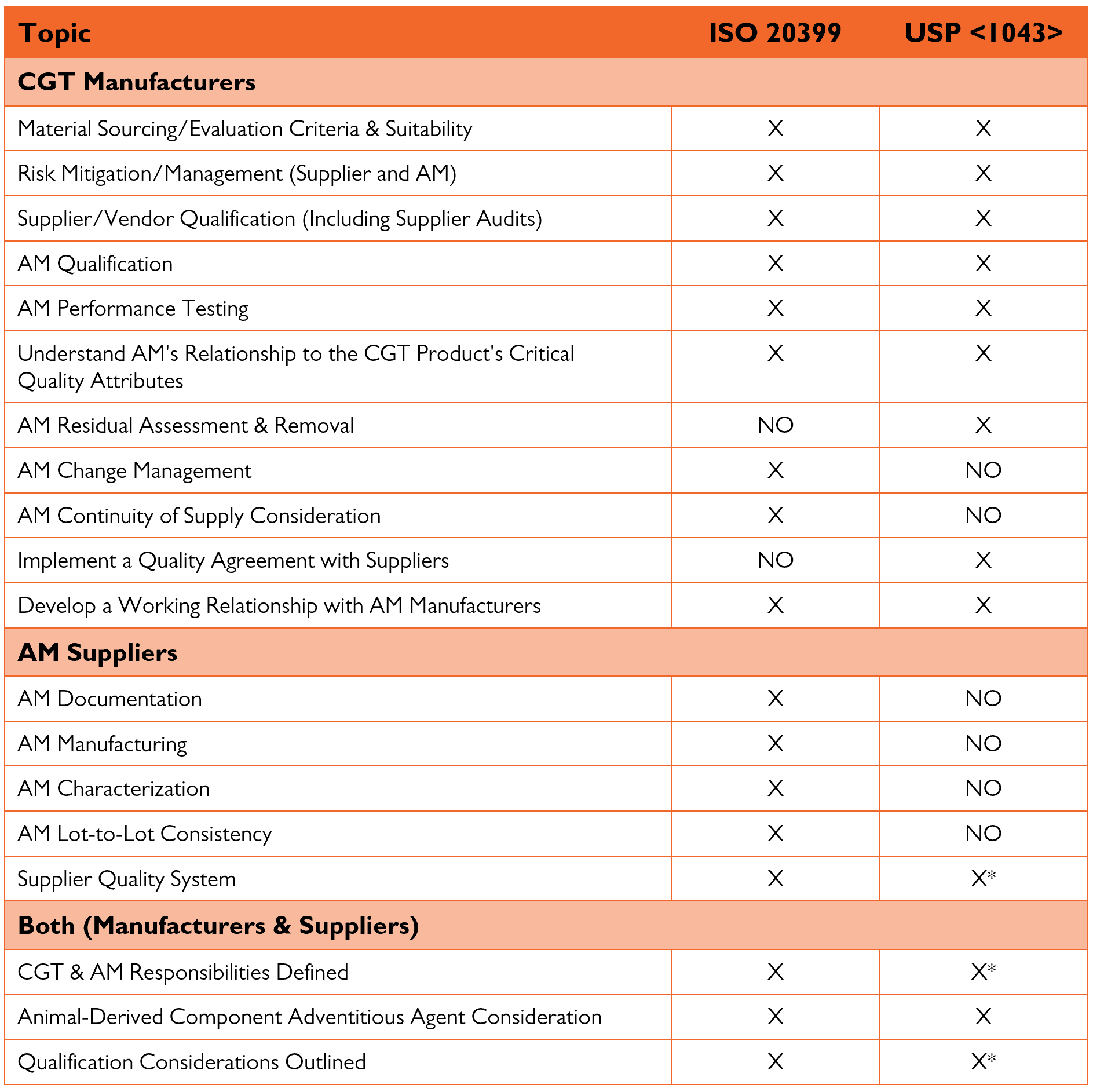
*Mentions, but no specifics provided
