
High Cost of Purified Collagenase
Clostridium histolyticum collagenase is a critical reagent for isolating islets from human pancreatic tissue. The development of Liberase HI Purified Enzyme Blend overcame the problem of repetitive evaluation of different lots of crude collagenase to identify an acceptable product. C. histolyticum class I (C1) and class II (C2) collagenases and neutral protease(s) were identified as the essential enzymes required for release of islets from pancreatic tissue (1).
The price of Liberase HI was ≈ 3-4 times the cost of an equivalent amount of crude collagenase. Three separate chromatography steps were required to isolate C1 from C2 collagenase (2). The final product contained ≈ 500 mg of a 60:40 C1:C2 ratio of purified collagenase and ≈ 11 mg of purified thermolysin. Liberase HI was the primary enzyme used for human islet isolation from 1994 to 2007. In 2007, NIH disallowed its use in the Clinical Islet Transplantation Consortium (CITC) trial because of the potential threat of transmission of bovine spongiform encephalopathy (3). Roche discontinued selling the product in 2008.
Nordmark/Serva was chosen as the sole enzyme vendor for the CITC trial. The price of GMP Grade NB-1 Collagenase was ≈ 2–3 times the price of the Liberase HI product. Many users found no difference in the performance of the GMP versus non-GMP Grade NB-1 Collagenase products. This led the majority of islet isolators to use the non-GMP, Premium NB-1 Collagenase, product that was priced similar to Liberase HI.
VitaCyte and Roche were added as suppliers for enzymes used in the CITC trial after several experienced islet transplant laboratories that used either grade of NB-1 Collagenase/NB Protease were unable to achieve human islet yields comparable to those obtained when using Liberase HI (4).
All the CITC trial enzyme formulations used about 20 Wünsch Units of collagenase per g of trimmed tissue, similar to the dose of Liberase HI used in the Immune Tolerance Network Trial for human islet transplantation (5). However, a retrospective analysis of human islet isolations at the University of Alberta showed that Wünsch activity (WU) did not correlate with the effectiveness of human islet isolation (6). Balamurugan et al confirmed this observation by showing a wide range of human islet yields after using enzyme mixtures from different suppliers at doses between 1600-4400 WU/isolation (4). They found the key variable associated with higher islet yields was the selection/dose of neutral protease and the use of collagenase enzyme products that contained primarily intact C1 collagenase. These results confirmed earlier reports that showed purified collagenase with multiple C1 peaks after anion exchange chromatography analysis were associated with lower human islet yields (7, 8).
Linking Experimental Results to Theory
Balamurugan’s observations (4, 8) can be explained by a hypothetical model of tissue dissociation reported earlier where the primary function of collagenase is to degrade collagen, loosening the extracellular matrix so that neutral proteases can accelerate the degradation of denatured collagen and extracellular matrix proteins (1, 9). The foundation for this model was based on results from Matsushita’s laboratory who showed class I (C1) and class II (C2) collagenase were expressed by separate genes (10), validating earlier observations that showed these two classes had different enzyme activity profiles and could be separated by chromatographic techniques (9). They also showed that C. histolyticum contained three separate protein domains: a catalytic domain that cut collagen, a linking domain of unknown function, and a collagen binding domain (CBD) which must bind to native collagen for the catalytic domain to cut native collagen. Intact C1 and intact C2 had four protein domains as shown in the Figure.
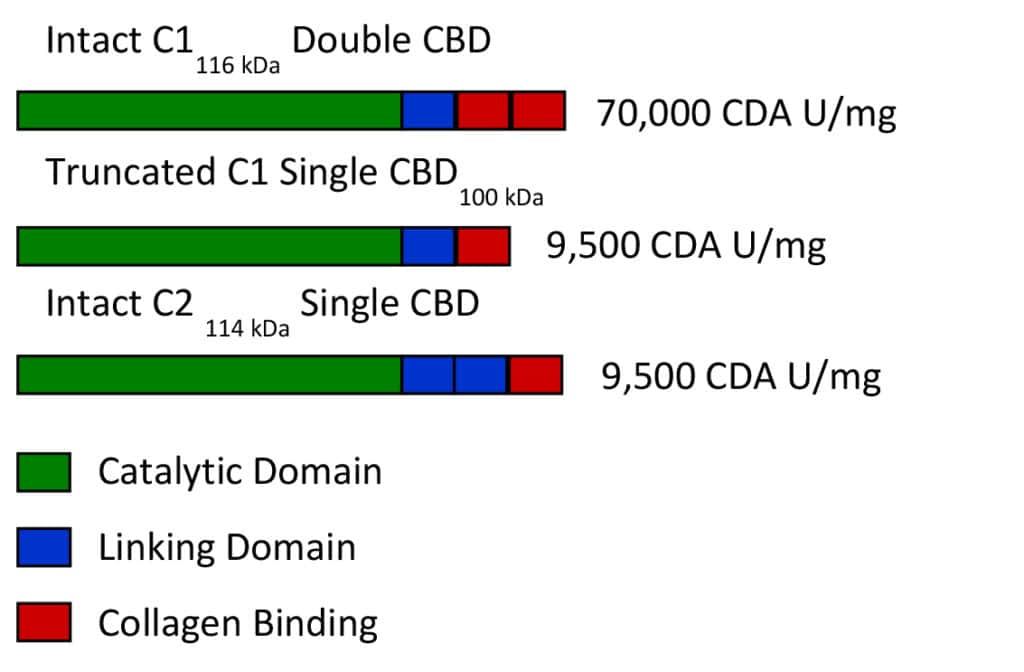
A third molecular form is also included in this Figure. Truncated C1 is a shorter molecular form of C1 since the carboxy terminal collagen binding domain is lost by proteolysis.
All the forms are termed functional collagenase since they all can degrade native collagen. Non-functional collagenase are those molecular forms that have lost their collagen binding domains and are unable to degrade native collagen. However, both functional and non-functional forms of collagenase have similar Wünsch and gelatinase activity since these activities require only a functional catalytic domain (1, 9).
As shown above, the specific CDA (CDA U/mg protein) is dependent upon the number of collagen binding domains. These data were derived from an experiment where peak fractions of purified collagenase were collected and then analyzed by SDS-PAGE or assay of CDA using a kinetic fluorescent microplate assay (11). Purified intact C1 with two collagen binding domains was 7-10 times more active in degrading native collagen when compared to “truncated” C1 or intact C2, each having only one collagen binding domain.
Six Key Principles from the Hypothetical Model are:
- Differences in the efficiency of intact C1 vs truncated C1 or intact C2 in CDA assays likely reflects the ability of intact C1 with two collagen binding domains to stick tighter to the collagen monomer during the degradation process than those forms with a single collagen binding domain.
- Collagenase appears to degrade collagen by moving from the amino terminal end of the collagen monomer to the carboxy terminal, degrading a collagen monomer in a fiber or fibril.
- Excess collagenase CDA must be used to ensure rapid degradation of collagen; excess purified collagenase will have a minimal adverse effect on cells since it has a narrow specificity for native collagen and gelatin.
- Collagenase products that contain predominantly intact collagenase will have the highest specific CDA, and can be used at lower doses in the islet isolation procedure than those products that contain truncated C1 or non-functional collagenase where the collagen binding domains are lost by proteolysis.
- The ratio of C1 to C2 is unlikely to impact the efficiency of degradation of collagen in the extracellular matrix since these two enzymes work cooperatively to degrade native collagen.
- Choice and dose of neutral protease used for the isolation procedure is the critical variable that must be controlled if excess intact collagenase is used in the isolation procedure.
Reducing Costs of Human Islet Isolation with Enriched Collagenase
The new understanding of the role collagenase plays in tissue dissociation leads to an obvious question, will a lower cost, high quality, enriched collagenase perform as well as higher priced, purified collagenase products? Khiatah and colleagues answered this question when they found that Collagenase Gold-BP Protease (Gold-BPP) enzyme mixtures were as effective as the higher priced, purified enzyme mixtures for human islet isolation (12). They emphasize that the Gold-BPP enzyme mixtures cost 54% and 23% of the Roche MTF and Serva NB-1 Collagenase/NB Protease products, respectively.
A recent report by Loganathan et al. confirmed Khiatah’s results and showed that the biochemical characteristics of the Gold enzyme were comparable or superior to purified collagenase enzymes manufactured by VitaCyte, Roche, or Nordmark (13). Gold contained primarily intact C1 and C2 as shown by anion exchange chromatography analysis and had high specific CDAs. Loganathan also showed that DE Collagenase 800 (DE 800) was as effective as Gold in human islet isolation. DE 800 contains enriched collagenase and purified BPP. DE 800 has about 10-15% more protease activity than Gold-BPP enzyme mixtures used by Khiatah et al or Loganathan et al.
The Gold and DE 800 products are stabilized by the addition of a non-mammalian, polypeptide excipient. There is no proteolytic degradation of the lyophilized products at elevated temperatures as reflected by anion exchange chomatography analysis or minimal loss of CDA. These data support the claim that these products can be shipped at ambient temperatures. The excipient also improves product handling since the amount required can be weighed out immediately before use. The product readily dissolves and is easily sterilized by passage through a 0.22 μm filter.
VitaCyte has recently introduced Collagenase Gold + (Gold +), a GMP Grade version of Collagenase Gold. Gold + is as stable as the Collagenase Gold but it does not contain an excipient. This product is reconstituted with water or buffered salt solution before use and any excess material can be frozen in tightly sealed storage containers. The Gold + product is assayed for CDA, FALGPA activity, and endotoxin contamination.
We believe the Gold or Gold + products are interchangable and can be used for research or clinical applications. For more information on the Collagenase Gold, DE Collagenase 800, or Collagenase Gold + products, visit the products page on VitaCyte’s website.
Reference
1. McCarthy RC, Green ML, Dwulet FE. Evolution of enzyme requirements for human islet isolation. OBM Transplantation [Internet]. 2018 2018 14 November 2018]; 2(4):[1-30 pp.]. Available from: http://www.lidsen.com/journals/transplantation/transplantation-02-04-024.
2. Lakey JR. Investigating the standardization and effective use of Roche Liberase collagenase blends. http://icrcohorg/docs/PDF%20Powerpoint%20Conversions/Lakey%20Collagenase%20Slidespdf. 2005 2005.
3. ElSaadany S. Preliminary quantitative risk assessment of developing variant Creutzfeldt-Jakob Disease (vCJD) from human islet transplantation. 2008 [Available from: http://www.med.uottawa.ca/sites/tsalem/documents/Islet%20Risk%20Assessment%20-%20Feb%2018,2008%20-%20final.pdf.
4. Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012 Apr 15;93(7):693-702. PubMed PMID: 22318245. PMCID: PMC3314155. Epub 2012/02/10. eng.
5. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation.[see comment]. New England Journal of Medicine. 2006 2006 Sep 28;355(13):1318-30.
6. Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. American Journal of Transplantation. 2007 2007 May;7(5):1233-41.
7. Barnett MJ, Zhai X, LeGatt DF, Cheng SB, Shapiro AMJ, Lakey JRT. Quantitative assessment of collagenase blends for human islet isolation. Transplantation. 2005 2005;80(6):723-8.
8. Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010 2010;89(8):954-61.
9. McCarthy RC BA, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: Factors to consider in setting enzyme acceptance criteria. Transplantation. 2011 2011;91:137-45.
10. Matsushita O, Okabe A. Clostridial hydrolytic enzymes degrading extracellular components. Toxicon. 2001 2001 Nov;39(11):1769-80.
11. McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, et al. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplantation proceedings. 2008 2008;40(2):339-42.
12. Khiatah B, Tucker A, Chen KT, Perez R, Bilbao S, Valiente L, et al. Evaluation of collagenase gold plus BP protease in isolating islets from human pancreata. Islets. 2018 Mar 4;10(2):51-9. PubMed PMID: 29381419. PMCID: PMC5895173. Epub 2018/01/31. eng.
13. Loganathan G, Hughes MG, Szot GL, Smith KE, Hussain A, Collins DR, et al. Low cost, enriched collagenase-purified protease enzyme mixtrues successfully used for human islet isolation. OBM Transplantation. 2019;3(2). Epub 2019.
