
“Control” is the watchword commonly used to describe a robust manufacturing process. The Clostridium histolyticum collagenase enzymes used for cell isolation are critical process parameters for cell manufacturing. Control of these enzymes can be challenging because their consistency depends on the manufacturing process used to generate these products.
Crude collagenase products were first sold in the 1960s, followed by enriched products that contained a higher amount of collagenase. The enzyme activities found in these traditional products reflect those present in the C. histolyticum culture supernatant at harvest time. These supernatants contain variable amounts of protease activity that can degrade the collagenase enzymes during fermentation and subsequent manufacturing steps. Proteolysis leads to a decrease or loss of collagen degradation activity that is critical for recovering cells from tissue or solid matrices. The lack of control in the collagenase manufacturing processes makes each lot of collagenase unique. Most end users “pre-qualify” lots before routine use. This process aims to select the lot that provides cells similar to those obtained with the current or earlier lots of collagenase. Once a “good” lot is identified, a large amount of this lot is purchased, lasting for a year or more.
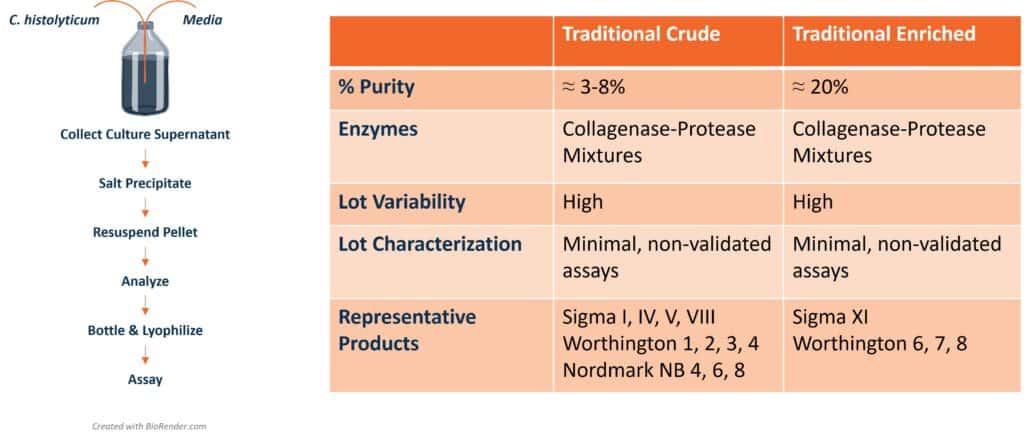
The inconsistency of traditional collagenase products hindered the translation of islet transplantation from animal models to clinical research. To overcome this hurdle, an R&D group at Boehringer Mannheim Biochemicals in Indianapolis identified the key enzymes in “good” lots of crude collagenase. This research led to the manufacture of Liberase™ HI Purified Enzyme Blend that contained purified class I and class II C. histolyticum collagenase and Thermolysin, a purified bacterial neutral protease.1
Many islet transplantation centers readily adopted Liberase HI since it avoided the costly lot qualification process described above. However, within ten years after launch, several experienced islet transplantation centers had difficulty recovering sufficient islets for transplantation. This problem coincided with a change in the collagenase manufacturing process. Enzymatic and analytical chromatographic analysis showed that increased proteolytic degradation of collagenase resulted in significantly lower human islet yields when compared to purified collagenase-protease enzyme mixtures containing predominantly intact collagenase. 1,2
The manufacture of purified collagenase enzymes with consistent specific collagen degradation activities (CDA U/mg protein) require reduction of the proteolytic degradation of collagenase during the fermentation and manufacturing process. The tighter control of these manufacturing processes created a new collagenase product category: purified-defined collagenase products. For these products, the relative concentrations of the different molecular forms of collagenase are determined by anion exchange chromatographic analysis (see US Pharmacopeia Chapters <89.1> and<89.2>) and assay of the collagen degradation and protease activities determined by using a kinetic fluorescent microtiter plate assay developed at VitaCyte.1,3
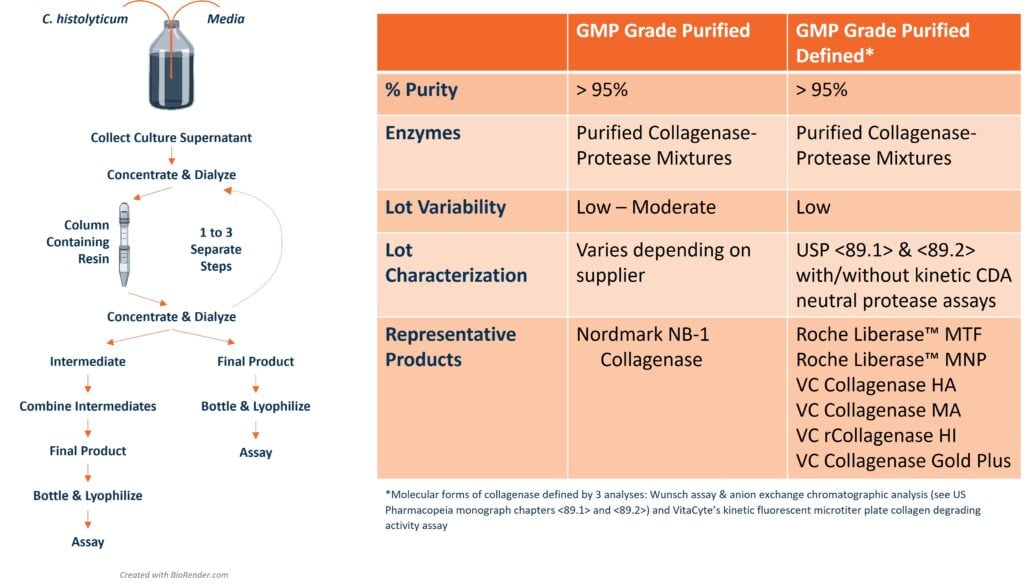
The lessons learned from identifying differences between purified vs. purified-defined collagenase:
- Purified does not guarantee consistency since proteases present in the raw material can degrade collagenase during the manufacturing process, affecting its ability to degrade native collagen
- Collagenase degradation is reduced if protease activity is minimized in the C. histolyticum culture supernatants and proteases are removed or are inactive during critical purification steps
- Control of manufacturing requires the use of appropriate analytical methods during the development of the manufacturing process to verify minimal degradation of collagenase
- The same analytical methods used above guides product specifications, ensuring the consistency of collagenase degradation activity, which is the only accurate measure of collagenase activity.
References
- McCarthy RC, Green ML, Dwulet FE. Evolution of Enzyme Requirements for Human Islet Isolation. OBM Transplantation 2018;2(4):024; doi:10.21926/obm.transplant.1804024.
- A. N. Balamurugan, Andrew G. Breite, Takayuki Anazawa, Gopalakrishnan Loganathan, Joshua J. Wilhelm, Klearchos K. Papas, Francis E. Dwulet, Robert C. McCarthy, Bernhard J. Hering. (2010). Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 89(8): 954–961.
- McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, et al. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplant Proc. 2008;40(2):339-42.
