Hypothetical Mechanism of Enzymatic Tissue Dissociation
From the initial use of traditional collagenase products in the 1960s for enzyme-mediated cell isolation, knowledge of the composition and dose of the enzymes required for successful cell isolation was unknown. In the mid-1990s, the development of the Liberase™ HI Purified Enzyme Blend for human islet isolation at Boehringer Mannheim Biochemicals showed that at least three enzymes were responsible for tissue digestion: Clostridium histolyticum class I (C1) and class II (C2) collagenase at least one or more neutral proteases. Ninety-eight percent of the protein mass in this product was collagenase.
Collagen: A Protease-Resistant Substrate – C. histolyticum Collagenase: Unique Collagen Degrading Enzymes
Collagen is the most abundant mammalian protein (≈ 30% of total protein) that provide structural support of tissue. It is highly conserved, containing a triple helical protein structure that is resistant to degradation by most proteases. Those proteases that degrade native collagen are termed collagenases. C. histolyticum collagenases have been used since the 1960s for enzyme-mediated cell isolation procedures. Their broad specificity to degrade nearly all types of mammalian collagen ensures the release of individual cells from tissue.
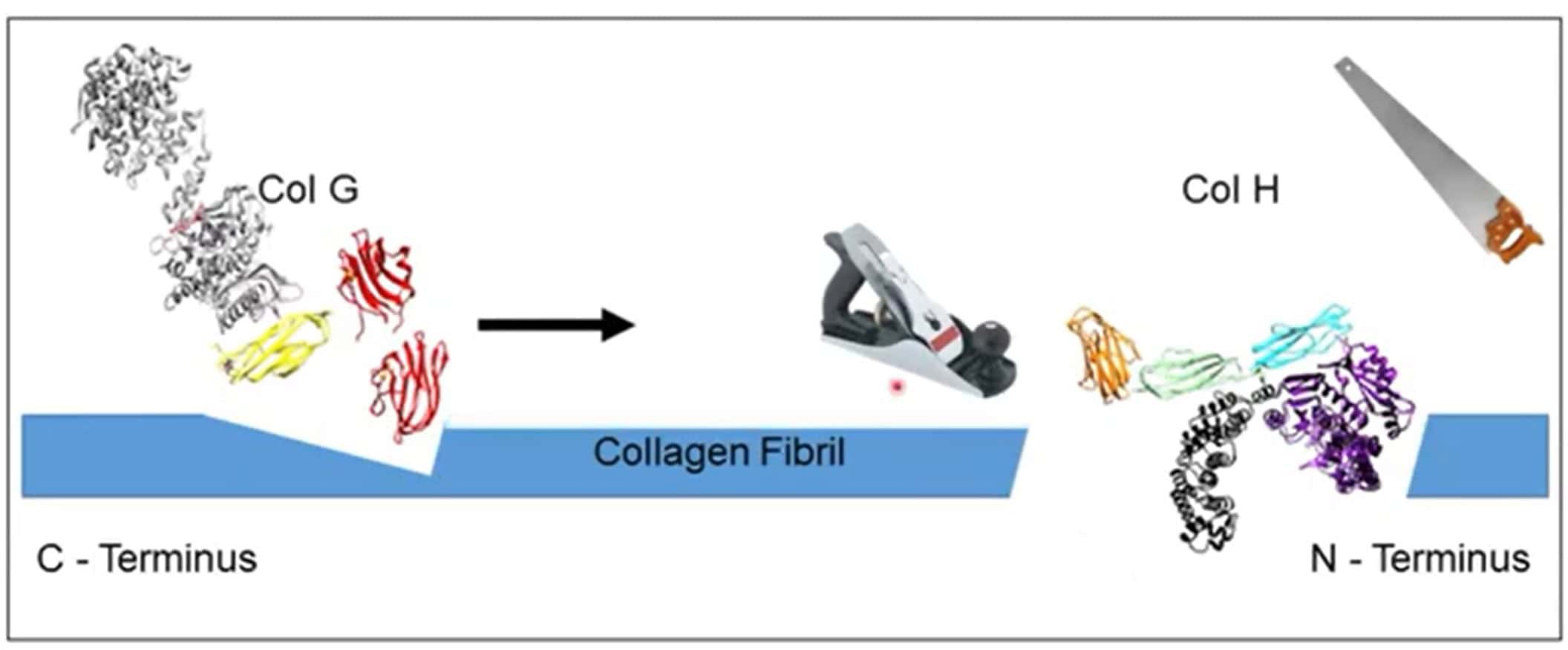
Illustration Source: Collagen Degradation by Clostridial histolyticum Collagenases – Josh Sakon
Sequencing of the C1 (colG) and C2 (colH) C. histolyticum collagenase genes, followed by structure-function studies of the expressed Col G and Col H enzymes showed that collagen degradation requires these enzymes to contain a catalytic domain and at least one collagen binding domain. Recent studies have shown that C1 is a processive protease where the collagen-binding domain(s) binds to collagen; it progresses from the carboxy end to the amino-terminal end of the protein, “pulling” the catalytic domain along. As this “catalytic caboose” moves along the protein, it degrades the “helical tracks.” C1’s activity is complemented by C2, an endoprotease that makes internal cuts in tropocollagen. Only intact C2, which contains a catalytic and a collagen-binding domain, can perform this function.
How Collagenase-Protease Mixtures Degrade Collagen
The improved understanding of collagenase function led to the development of a hypothetical model for enzyme-mediated tissue dissociation.
Axioms That Led to the Hypothetical Model
- Purified histolyticum collagenase has a narrow selectivity for collagen or gelatin (denatured collagen)
- Excess purified collagenase will have a minimal adverse effect on cell viability or function because of its restricted enzyme selectivity for collagen, the predominant protein in the extracellular matrix (ECM)
- The selection and dose of protease is the critical enzymatic activity that must be controlled to ensure the release of viable, functional cells from tissue
- The dose of neutral protease used for most cell isolations is excessive, as shown by the optimization of several purified-defined collagenase-protease enzyme mixtures used to isolate human islets or rodent hepatocytes
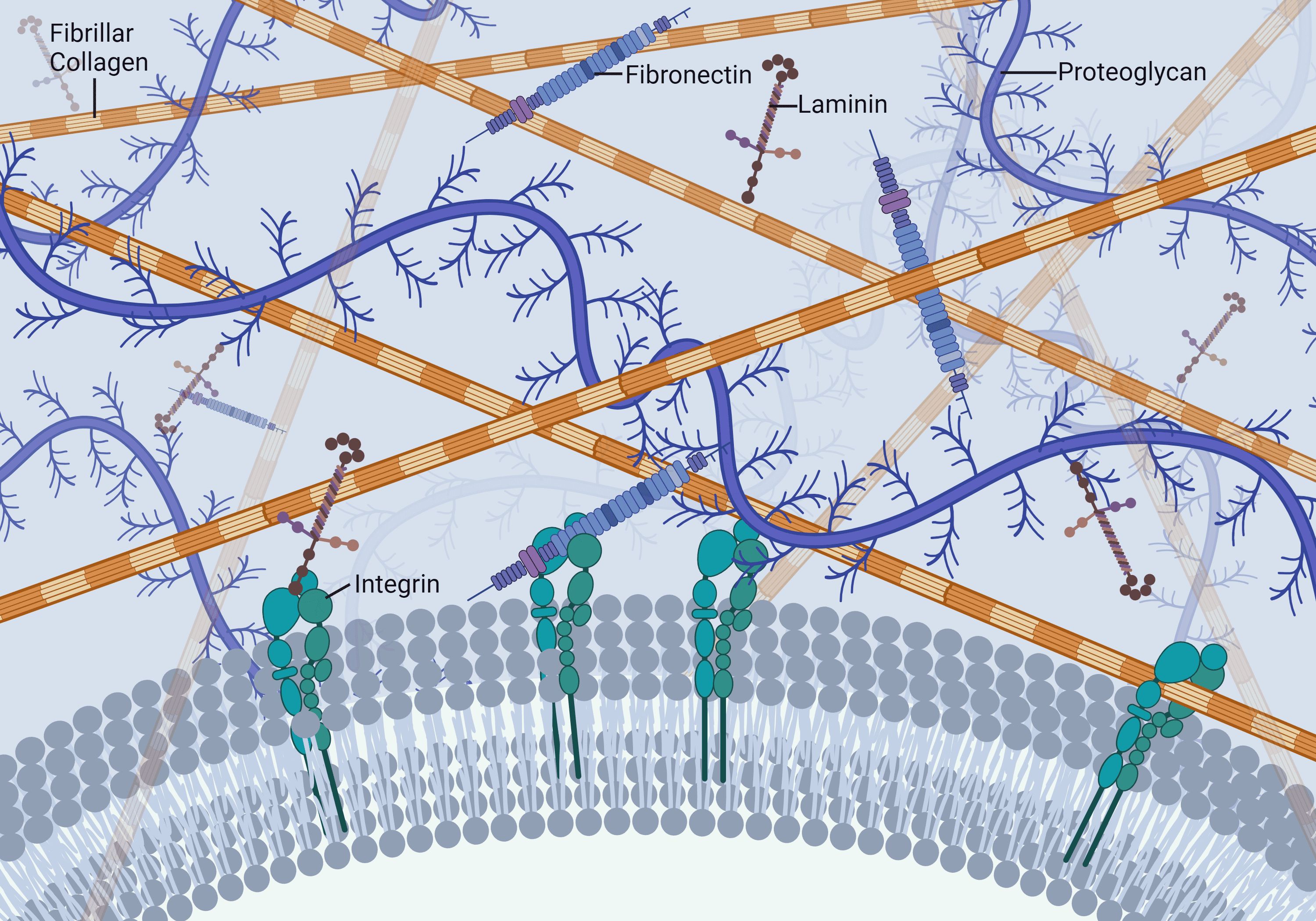
- Cells are tethered to tissue by cell anchoring proteins within the ECM, which are directly or indirectly associated with the collagen fibrils or fibers
- Collagen acts as a protease-resistant shield, protecting the ECM proteins from proteolytic degradation
- Proteases alone are unable to free cells from tissue, so enzyme mixtures must contain a sufficient amount of collagenase’s collagen degradation activity to free cells from tissue
- Collagenase degrades collagen by the synergistic attack of C1 and C2 collagenase on individual tropocollagen molecules
- Collagenase disrupts the ECM, leading to exposure of protease-sensitive sites on the ECM proteins
- Proteases cleave these exposed sites, leading to breaking the molecular tethers that hold cells to the ECM, freeing individual cells or cellular aggregates from tissue
Why Does This Matter?
The true measure of collagenase function is collagen degradation activity
-
- There is no correlation between peptidase (Wünsch or FALGPA activity) and collagen degradation activity
- Wünsch or FALGPA activities detect C2’s endoprotease activity where a functional catalytic domain (no collagen binding domains) has peptidase activity
Understanding the biochemical characteristics of collagenase and the mechanism of action for collagenase-mediated cell isolation is critical
-
- If collagenase’s collagen degradation activity is in excess, then it is the neutral protease that determines the success of cell recovery
The model above explains why lot pre-qualification is required before using traditional crude and enriched collagenase enzymes for enzyme-mediated cell isolation
-
- The balance of collagenase and protease activities in these products is unknown
- Protease degradation of collagenase affects collagenase’s collagen-degrading activity but does not affect peptidase activity (e.g., Wünsch or FALGPA activities)
Deep understanding of the model enhances the end user’s control of an enzyme-mediated cell isolation procedure
-
- Commonplace to “blame the enzyme” for failure of an isolation procedure when it should be the most tightly controlled reagent essential for tissue dissociation, a critical process for cell isolation
- Ruling out the enzyme as a potential cause of failure leads to end user to focus on other factors responsible for failure