Competitive and Technical Comparisons of Collagenase Products
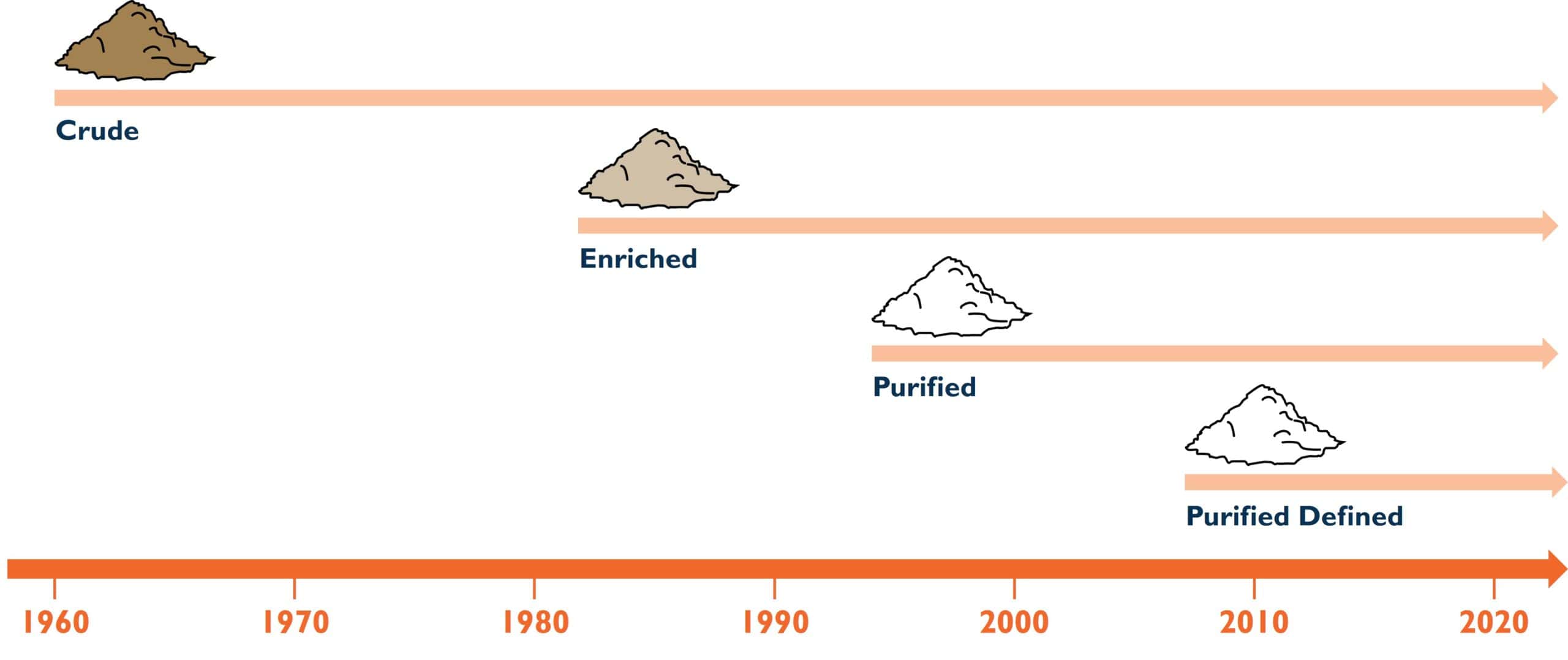
A historical perspective can divide collagenase products into three different categories based on enzyme purity and characterization of the collagenase activities.
Traditional Crude or Enriched Collagenase Products
Purified Collagenase Products
Purified-Defined Collagenase Products
Traditional crude and enriched collagenase enzymes are minimally processed cell culture supernatants recovered after anaerobic fermentation of Clostridium histolyticum. To reduce the volume of the fermentation supernatant and to enrich the collagenase activity in the final product, the proteins in the culture supernatant are precipitated with saturated salt solution. The precipitated protein is then resuspended in a smaller volume, and extensively dialyzed to remove low molecular weight contaminants. Additional steps are taken to further enrich the collagenase activity, often leading to a tan lyophilized protein product.
Crude Manufacturing Process
Crude or enriched collagenase products contain only a small percentage of collagenase. Subsequent development of purified collagenase products showed that class I (C1) class II (C2) and one or more neutral proteases are required for tissue dissociation. For crude and enriched collagenase C. histolyticum neutral protease and clostripain provide this activity. Collagenase and neutral protease activity will differ depending on the fermentation conditions making each lot unique. This “uniqueness” led many suppliers to offer collagenase lot qualification programs, enabling users to assess the performance of several different lots of collagenase in their cell isolation procedure before their current lot of product is depleted. Once a “good” lot is identified that performs similarly to the current lot, then a larger amount of this specific lot of collagenase is purchased to last a year or more.
In 1995, Liberase HI Purified Enzyme Blend was introduced at the first purified collagenase-protease enzyme mixture designed for human islet isolation. Several chromatographic steps were used to first separate class I (C1) and class II (C2) collagenases from other proteins with the final step chromatographic step separating C1 from C2. The C1 & C2 intermediates were then mixed in a 60:40 ratio with a small amount of Thermolysin, the product frozen, lyophilized, and bottled.
Evolution of Purified Collagenase Enzymes
For many years, Liberase HI was the “gold standard” for human islet isolation but a change in the manufacturing process after 2002 resulted in inconsistent recoveries of human islets. The same problem was encountered when another purified collagenase enzyme replaced Liberase HI in an NIH funded clinical trial to show the benefit of human islet allotransplantation to treat adult brittle type 1 diabetic patients. VitaCyte analyzed these problematic enzymes and showed that suboptimal islet yields could be explained by degradation of the collagenase by proteases. The use of a kinetic, fluorescent microtiter plate collagen degradation activity (CDA) assay was critical to reach this conclusion since these results showed that the problematic collagenases contained partially degraded C1 that had a lower specific CDA (CDA U/mg protein) than intact C1. This difference in CDA correlated with lower islet yields when a modified a Ricordi procedure was used to prepare islets for this clinical trial.
The importance of CDA to assess collagenase function is not surprising since it is the only true measure of collagenase function: degradation of native collagen. The first CDA assay was described in 1952 measuring the degradation of Achilles tendon collagen fibers. The Mandl CDA assay was a seven hour research assay but it was never rigorously characterized.
By contrast, the kinetic fluorescent microtiter plate CDA assay provides a precise analysis of CDA (between run CV < 15%) and the assay can distinguish between the functional forms of collagenase: intact C1 (116 kDa), truncated C1 that lost a carboxy terminal collagen binding domain by proteolysis (100 kDa), and intact C2. The table below summarizes the CDA specific activities generated by the kinetic microtiter plate CDA.
Purified Manufacturing Process

| Intact C1 116 kDa | Truncated C1 100 kDa | Intact C2 114 kDa | |
|---|---|---|---|
| CDA U/mg protein (n) | 134,739 (20) | 53,185 (12) | 13,320 (15) |
| Normalized as % intact C1 | 100 | 39.5 | 9.9 |
The table below summarizes the collagenase products sold on the market today. It does not include specialized products designed for specific cell isolation applications. The primary difference between the purified and purified-defined products is the rigorous characterization of the purified-defined enzymes. These enzymes are assayed for CDA using a kinetic, fluorescent microtiter plate assay. The information presented above showed that each of the three active forms of collagenase have a unique specific CDA (CDA U/mg protein). This conclusion was determined by taking peak fractions of the C2, C1, C1b, and C1c peaks recovered after analytical anion exchange chromatography and analyzing the collagenase in each peak fraction for molecular mass using SDS-polyacrylamide electrophoresis and specific CDA using the kinetic microplate CDA described above. The conclusion from this analysis led VitaCyte to recommend that purified collagenases should be analyzed by anion exchange chromatographic analysis, specific CDA by the kinetic microplate assay, and for specific Wunsch activity. Anion exchange chromatographic analysis detects degradation of C1 collagenase. Results from the analytic anion exchange analysis are aligned with the specific CDA where preparations containing truncated C1 (i.e., C1b or C1c peaks) have lower specific CDAs. The specific Wunsch activity provides an estimate of C2 in the collagenase product but does not correlate with results from the CDA assay.
| Collagen Degradation Activitya (CDA) (Assay) |
Peptidase Activityb (Substrate) |
Protease Activityc ± to ++++ |
Re-Blending of Purified C1 and C2d (C1:C2 ratio) | Anion Exchange Analysise | Assay for Endotoxinf | |
| Crude Collagenase (3-8% collagenase) | ||||||
| Worthington Types 1, 2, 3, 4 | Y (Mandl) | ND | ++ to +++ | N | N | N |
| Sigma I, IA, II, IV, V, VIII | Y (Mandl) | Y (FALGPA) | ++ to +++ | N | N | N |
| Enriched Collagenase (15-25% collagenase) | ||||||
| Worthington Types 5, 6, 7 | Y (Mandl) | ND | ++ to +++ | N | N | N |
| Sigma XI | Y (Mandl) | Y (FALGPA) | + to +++ | N | N | N |
| Nordmark NB-4, NB-5, NB-6, NB-7 | N | Y (Pz peptde) | ++ | N | N | N |
| Purified Collagenase (>90% collagenase) | ||||||
| Worthington CLPSA, CLSAFB, CLSAFC, CLSAFD | Y (Mandl) | N | ? | N | N | N |
| Sigma Types III, VII | Y (Mandl) | Y (FALGPA) | ++ | N | N | N |
| Roche Liberase* TL, TM, TH, and DH | N | Y (Pz peptide) | ++ to ++++ | Y (60:40) | Y | Y |
| Nordmark AF-1, NB-1, NB-8 | N | Y (Pz peptide) | + | N | N | Y |
| Purified-Defined Collagenase (>95% collagenase) | ||||||
| VitaCyte Collagenase HA, MA | Y (MTP) | Y (Pz peptide) | ± | Y (60:40) | Y | Y |
| VitaCyte rCollagenase HI | Y (MTP) | Y (Pz peptide) | ± | Y (50:50) | Y | Y |
| VitaCyte Collagenase Gold | Y (MTP) | Y (FALGPA) | ± | N | Y | N |
| VitaCyte Collagenase Gold Plus | Y (MTP) | Y (Pz peptide) | ± | N | Y | Y |
aTwo CDA assays have been described, the Mandl assay (1953) or MTP (kinetic fluorescent microtiter plate assay)
bPeptidase substrate: measures C2 activity using the FALGLA substrate or PZ peptide the substrate used in the Wünsch assay. The standard Wünsch assay is described in USP monograph chapters <89.1> or <89.2>
cProtease activity is presented as a relative measure from minimal (±) to low (+) to high (++++) since there is no standard assay to measure this activity.
dRoche and some VitaCyte products are manufactured with a 60:40 C1:C2 ratio whereas for other products (VitaCyte Gold products and Nordmark’s purified collagenase products) the C1 and C2 are not separated from each other.
eAnalytical anion exchange chromatographic analysis described in described in USP monograph chapters <89.1> or <89.2>
fEndotoxin contamination detected using standard Limulus based assay
The primary limitation of using traditional crude or enriched collagenase products is that the only adjustment the cell isolator can make is changing the enzyme dose used in the isolation procedure. The enzyme composition is unknown and the ratio of collagenase to protease activity fixed. In contrast, the use of purified collagenase and purified enzymes enables cell isolators to modify the enzyme compositions by evaluating the dose of collagenase or protease on the recovery of viable functional cells. VitaCyte rigorous characterization of its enzyme products ensures that the same collagenase and protease activites will be obtained every time.
