Importance, Measurement & Mechanism for Cell Isolation
Presented by
Robert McCarthy, VitaCyte LLC
Joshua Sakon, University of Arkansas
Collagen degradation activity (CDA) should be the best measure of C. histolyticum collagenase activity required to release cells from tissue. Sigma and Worthington Biochemicals use the Mandl assay to measure collagenases’ degradation of collagen fibers from the Achilles tendon. However, this research assay, developed in the 1950s, has never been characterized. No reports discuss the linearity, precision, or specificity of this assay nor define the effectiveness of class I or class II collagenase to degrade collagen fibers.
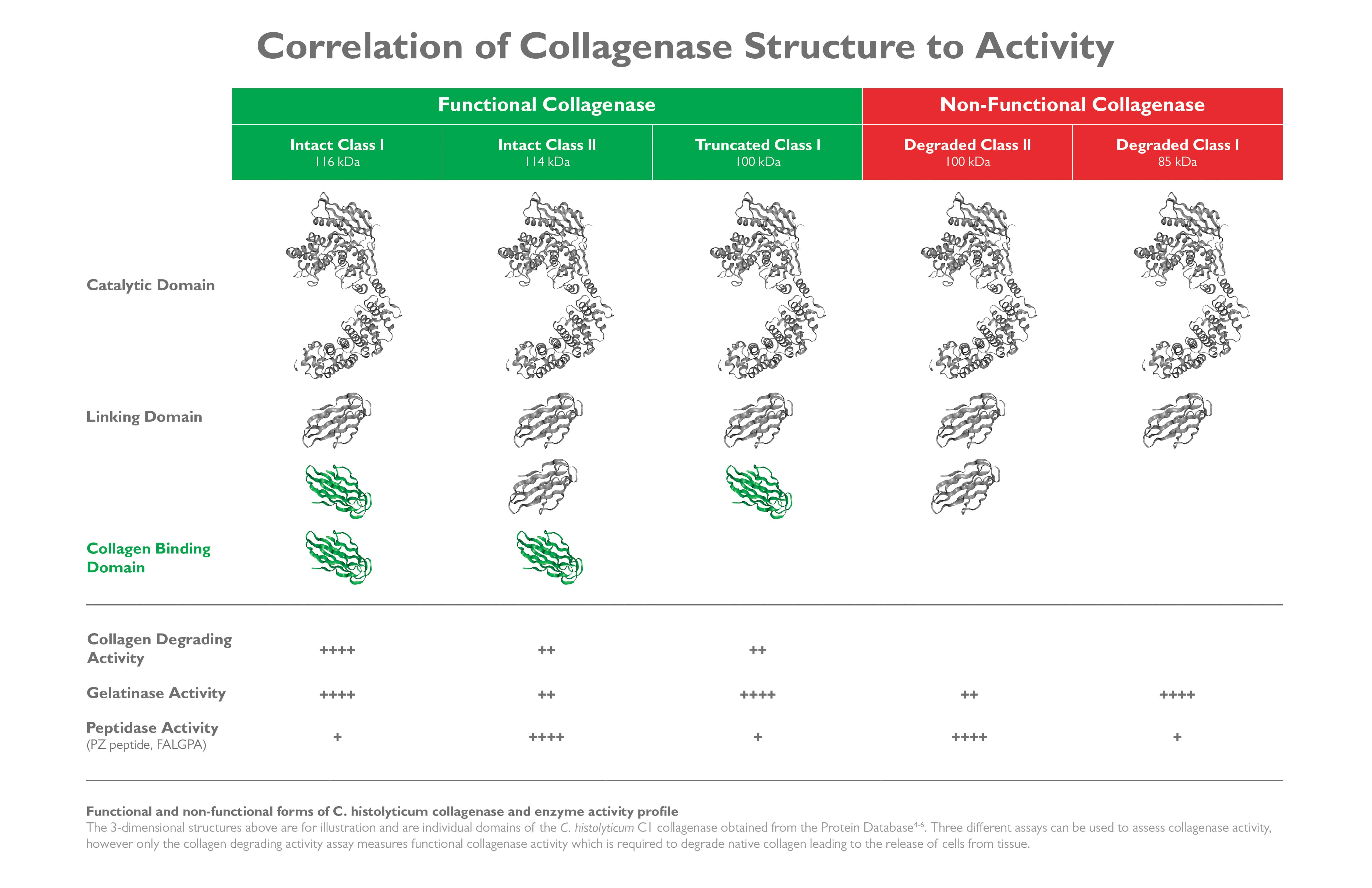
In 2008, RC McCarthy et al. developed a kinetic, fluorescent, microtiter plate CDA. Here, collagenase unwinds/degrades a fluorescein isothiocyanate-bovine collagen fibril substrate (FITC fibrils), leading to an increase in fluorescence. Collagenase-protease enzyme mixtures with higher specific CDA activity (CDA U/mg purified collagenase) gave higher islet yields than those containing lower specific CDA. This difference reflected the loss of the carboxy-terminal collagen-binding domain on intact class I collagenase. Intact class I collagenase with two collagen-binding domains had 8-10 fold higher specific CDAs than intact class II collagenase or truncated class I collagenase with only one collagen-binding domain. Class I and class II collagenase are needed for the synergistic degradation of collagen.
How do we explain how these two classes of collagenases degrade native collagen?
In 2003, Josh Sakon’s group at the University of Arkansas began performing structure-function studies of different domains of C. histolyticum class I and class II collagenase. Sakon’s initial work focused on the binding of collagenase’s collagen-binding domain to collagen, with the ultimate goal to understand how class I and class II collagenase enzymes degrade native collagen.
Bob McCarthy will review the fundamental aspects of C. histolyticum collagenase biochemistry and the current state of CDA assays, including the development of a reliable Mandl assay. Josh Sakon will finish the webinar by discussing the current thinking about how these enzymes degrade native collagen.
Correlation of Collagenase Structure to Activity Poster
Meet the Presenters

Robert McCarthy
VitaCyte LLC
Bob McCarthy received post-graduate training in immunology and joined Boehringer Mannheim Biochemicals in Indianapolis in 1987. In 1992, he led the R&D team that developed the Liberase HI Purified Enzyme Blend product to improve human islet recovery. In 2004, Francis Dwulet and Bob co-founded VitaCyte to improve the manufacture and characterization of collagenase and protease enzymes used for cell isolation. VitaCyte’s mission is to develop products that result in increased research productivity or improved cell therapy outcomes.

Joshua Sakon, Ph.D.
University of Arkansas
Joshua Sakon, Ph.D. is a Professor at University of Arkansas. His curiosity in crystallography and enzymology has resulted in 15 patents, which several have been company licensed, and over 90 articles published. His involvement in industry has led him to be a founder of two companies, BiologicsMD LLC (VC funded) and S3 Biologics LLC (launched in 2021). Joshua is active in both local community events and international research organizations, including Advances in Mineral Metabolism. He is an avid runner and has run the Boston Marathon in two hours and thirty-six minutes.
